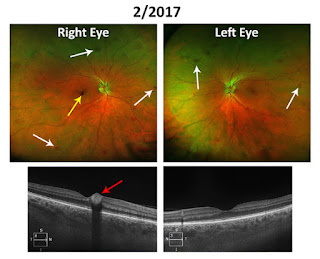
Familial Mediterranean Fever: Causes, Diagnosis, and Treatment
hemophilia b inhibitors :: Article Creator Gene Therapy For Hemophilia Becomes A Reality Earlier this year we reported on FDA's approval of valoctocogene roxaparvovec (Roctavian) for adults with severe hemophilia A. In this story, we provide an update on what has happened since. Two gene therapies have now been approved for the treatment of hemophilia following the FDA's June approval of valoctocogene roxaparvovec (Roctavian) as the first gene therapy for hemophilia A -- joining etranacogene dezaparvovec (Hemgenix) for hemophilia B, which was approved late last year. But uptake of these new therapies has been relatively slow. Hemophilia A Approval of valoctocogene roxaparvovec for adults with severe hemophilia A was based on data from the global phase III GENEr8-1 study, in which the therapy reduced the average annualized bleeding rate (ABR) by 52% compared with patients' baseline ABR on routine factor VIII prophylaxis. Mean ...