Glanzmann Thrombasthenia: treatment strategies | JBM
Pompe Disease
Channels
Content type
Categories
Categories
Sort by
Order
New Treatment Helps Symptoms Of Pompe Disease
LOS ANGELES Physical therapy is still a grind for Monique Griffin. Just two years ago, she was busy marketing casinos in Las Vegas. But in 2009 she hit a wall: She couldn't breathe or move."Nobody seemed to know what was wrong with me. They knew something was wrong with me at that point. I wasn't crazy," said Griffin.
She was diagnosed with Pompe disease, a form of muscular dystrophy. Her body stores too much sugar, which destroys her muscles.
"How much worse is it going to get? I'm having these problems breathing, I'm in unbearable pain," said Griffin.
"We need to combine strategies of different approaches to treating this muscle disease," said Dr. Barry Byrne, a pediatric cardiologist at the University of Florida.
Byrne was part of a team that developed the first and only clinical trial in the world to find a cure.
The result: Lumizyme infusions, which allow Griffin's body to process sugar. It was approved by the /*Food and Drug Administration*/ in the past year. The two-hour-plus process is still tough on patients.
"They have to be committed to it and that's certainly the case in all the patients I've encountered," said Byrne.
"Some people just call it stubborn," said Griffin. "And finally that personality trait is coming in handy."
If anybody's committed, it's Griffin. After daily workouts, doctors say she's starting to re-gain strength -- unheard of for her condition.
"These diseases, they just make you weaker and weaker and weaker, and it's very much a miracle to be able to take some of that back," said Griffin.
A small, but major, victory over a debilitating disease.
Griffin now writes a nationally recognized blog about Pompe disease.
Experts say occurrences are so low because Pompe disease mimics the symptoms of other forms of muscular dystrophy. Therefore, the disease is difficult to diagnose.
Pompe disease, according to the /*National Institutes of Health*/, is an inherited disorder most commonly caused by a buildup of sugar in the cells in the body. This buildup of sugar causes certain cells in the body to function improperly. There are three main types of Pompe disease:
Classic infantile-onset: This type begins shortly after birth, within a few months. Children and infants with this type of Pompe disease experience muscle weakness, poor muscle tone, an enlarged liver and heart defects. Another common effect from this type is that children experience a difficult time in growing and weight gain. Also, many infants experience classic infantile-onset with having breathing problems and difficulties.
Non-classic infantile onset: This type appears no later than age 1. Common symptoms of this type include: delayed motor skills and progressive muscle weakness. Oftentimes the heart is unusually large and the child experiences severe breathing problems. Children diagnosed with non-classic infantile onset do not live past early childhood.
Late onset: Late onset Pompe disease does not become apparent until late childhood, adolescence or even adulthood. The good news with late onset Pompe disease is that it does not attack the heart as severely as classic infantile and non-classic infantile. They do experience breathing problems, however, and respiratory failure is often the result.
TREATMENT: An enzyme replacement therapy has been created to help those suffering from Pompe disease. This enzyme therapy was developed to help decrease heart size, and reduce sugar accumulation. Myozyme is a drug recently approved by the Food and Drug Administration (FDA) for treatment of Pompe disease in infants and young children. For those adults experiencing late onset Pompe disease, the FDA has approved Lumizyme. (Source: National Institute of Neurological Disorders and Stroke)
PROGNOSIS: Typically, the later the onset of the disease, the slower the progression and the better off the patient is. Normally, the prognosis of the disease is determined by the extent of respiratory muscle involvement. For those babies diagnosed with classic infantile onset, the babies will die before the age of one from cardiac failure. Those with late onset, usually have a better chance of survival.
Interview with Dr. Barry Byrne, Pediatric Cardiologist at the University of Florida
What is Pompe disease?Dr. Barry Byrne: Pompe disease is a form of muscular dystrophy that affects all of the muscles in the body, particularly the heart muscle in infants, and the skeletal and respiratory muscles severely in adults. The form of weakness is so severe in children that some of the untreated ones don't live past their first year of life. So, it's one of the more severe forms of muscular dystrophy that we know about.
What other conditions is Pompe disease similar to?Dr. Barry Byrne: There is a class of muscular dystrophies called limb-girdle muscular dystrophies because of their distribution of weakness. So mostly in the trunk and girdle musculature -- around the legs and hips -- is the weakness in stabilizing the hip and influence in walking. Those types of weakness can be caused by approximately 40 different genetic causes, one of which is Pompe disease. Although it might fall under the classification of limb-girdle muscular dystrophy, it is slightly different in that the cause is not one of the structural proteins that make up the cell, but a protein that is involved in glycogen metabolism. Glycogen is a form of storage energy that is linked to one another, in order to be used to maintain the blood glucose. In patients with Pompe disease, this accumulates in one part of the cell, and causes this cell to work inefficiently. In some cases, these cells die, furthermore contributing to the weakness.
How has Pompe disease been treated in the past?Dr. Barry Byrne: The treatment for Pompe disease prior to 2002 was really all symptomatic; assistant devices for adults -- ventilatory assistance, wheelchairs -- were commonly provided. In infants, the progression of the disease was so severe that there really wasn't much that could be done besides managing the complications of the disease as best as one could. That was around the time that studies started in infants that investigated the protein -- which is now known as myozyme or lumizyme (the adult version of this drug) -- so as to replace the missing function of this protein in the cell, and moreover help cells metabolize glycogen.
Is the same drug for children administered to adults?Dr. Barry Byrne: Essentially it is the same drug manufactured in two locations, but with two different names. The pediatric version for children under eight -- particularly those with heart problems in the early onset of the disease -- is called myozyme. That was first approved in April 2006. Just this year, lumizyme -- a version that has been tested in adult subjects -- has been approved in the United States in addition to being approved in Europe.
What benefits have you seen?Dr. Barry Byrne: In Monique's case, she has actually been able to improve her activity level as a result she is able to exercise again. I think that this was very significant for her, because her overall health has improved chiefly because of it. I think that we all know the benefits of exercise in patients who do not have muscle disease, but even in those who have some primary muscle problem, the right type and amount of exercise can really benefit them overall. This is particular to their cardiovascular health, in addition to their overall wellbeing. Consequently, she feels a lot better, and that is the essential thing. She is able to swim now. What's more, she has gotten involved in some exercise classes. All of this will help her get back to work (which she loves), and be generally productive in her daily routine.
Is this considered "a cure" for all intents and purposes?Dr. Barry Byrne: Any medication that needs to be administered continually, and shows signs of reversing the symptoms would never be labeled as "a cure." It is a long step to understanding what the limitations are of an infused therapy like it. The current thinking though is that we need to combine strategies of different approaches in order to treat this muscle disease in a way that we get the overall best benefit. I don't think that any one approach is going to dominate the treatment regiment. If you think about that in other forms of medicine, we commonly combine therapies that complement one another. That is the approach that we are trying to take. Certainly when you are trying to sort out the effectiveness of one approach over the other, they must be done separately, however, that doesn't mean that after the demonstration of efficacy in one drug, another drug might not be added to aid in the overall treatment of symptoms.
Copyright © 2023 KTRK-TV. All Rights Reserved.
Pompe Disease Treatment Market Size, Share, Business Growth & Report 2023-2028
The global Pompe disease treatment market size reached US$ 1.01 Billion in 2022. Looking forward, IMARC Group expects the market to reach US$ 1.20 Billion by 2028, exhibiting a growth rate (CAGR) of 3.0% during 2023-2028. The increasing number of clinical studies and trials, the rising popularity of ERT, and the growing number of patients suffering from Pompe disease represent some of the key factors driving the market.
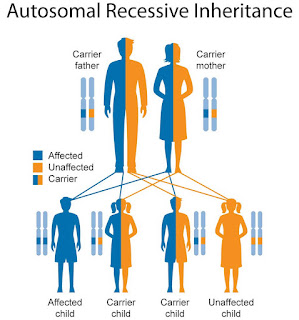
Pompe disease is a genetic disorder resulting from the accumulation of complex sugar (glycogen) in cells, affecting organs, tissues, and muscles. This condition is caused by a deficiency of the lysosomal enzyme acid alpha-glucosidase (GAA) and is inherited in an autosomal recessive manner. Symptoms of Pompe disease include muscle weakness, an enlarged liver, difficulty breathing, hearing problems, lung infections, irregular heartbeat, and weight loss.
Diagnosis of Pompe disease involves various tools and tests such as electromyography, X-rays, and magnetic resonance imaging (MRI). Fortunately, there are several treatment options available to improve the quality of life for those affected. These include medications, enzyme replacement therapy (ERT), substrate reduction therapy (SRT), chaperone-advanced replacement therapy (CART), and injections. By utilizing these treatments, individuals with Pompe disease can experience relief from their symptoms and enjoy an improved quality of life.
Request a Free Sample Report:- https://www.Imarcgroup.Com/pompe-disease-treatment-market/requestsample
Pompe Disease Treatment Market Trends and Drivers:The growth of the Pompe disease treatment market is primarily driven by the increasing prevalence of genetic abnormalities, which is heightened by growing concerns among parents about the health of their newborns. Additionally, the expanding geriatric population, more vulnerable to medical disorders, is also contributing significantly to market growth. Furthermore, the market is experiencing a boost from the rising number of clinical studies and trials aimed at developing new treatments.
The popularity of effective treatments such as stem cell transplant, bone marrow transplantation, chemotherapy, and immunotherapy is playing a crucial role in propelling the global market due to their numerous advantages. Moreover, the launch of favorable policies by government bodies and NGOs, coupled with continuous advancements in diagnostic technologies, is expected to further fuel the Pompe disease treatment market during the forecasted period.
Global Pompe Disease Treatment Market 2023-2028 Analysis and Segmentation:Top Key Players covered in this report are: Amicus Therapeutics Inc., Audentes Therapeutics Inc. (Astellas US Holding Inc.), Oxyrane UK Limited, Sanofi S.A., Spark Therapeutics Inc, etc.
Buy Complete Report:- https://www.Imarcgroup.Com/checkout?Id=6708&method=1
The report has segmented the market on the basis of region, treatment, route of administration, distribution channel, and indication type.
Treatment Insights:
The report has provided a detailed breakup and analysis of the Pompe disease treatment market based on the treatment. This includes enzyme replacement therapy (ERT), substrate reduction therapy (SRT), chaperone-advanced replacement therapy (CART), and others. According to the report, enzyme replacement therapy (ERT) represented the largest segment.
Route 0f Administration Insights:
A detailed breakup and analysis of the Pompe disease treatment market based on the route of administration has also been provided in the report. This includes oral, intravenous, and others.
Distribution Channel Insights:
A detailed breakup and analysis of the Pompe disease treatment market based on the distribution channel has also been provided in the report. This includes hospital and clinics, retail, and online pharmacies, and others. According to the report, hospital and clinics pharmacies accounted for the largest market share.
Indication Type Insights:
A detailed breakup and analysis of the Pompe disease treatment market based on the indication type has also been provided in the report. This includes infantile-onset Pompe disease (IOPD), classic infantile form, non-classic infantile form, late-onset Pompe disease (LOPD), and others. According to the report, late-onset Pompe disease (LOPD) accounted for the largest market share.
Regional Insights:
The report has also provided a comprehensive analysis of all the major regional markets that include North America (the United States and Canada), Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others), Europe (Germany, France, the United Kingdom, Italy, Spain, Russia, and others), Latin America (Brazil, Mexico, and others), and the Middle East and Africa. According to the report, North America was the largest market for Pompe disease treatment. Some of the factors driving the North America Pompe disease treatment market included the growing awareness about various alternative treatments, increasing number of clinical studies and trials, and well-established healthcare facilities.
If you want latest primary and secondary data (2023-2028) with Cost Module, Business Strategy, Distribution Channel, etc. Click request free sample report, published report will be delivered to you in PDF format via email within 24 to 48 hours of receiving full payment.
Key highlights of the report:If you need specific information that is not currently within the scope of the report, we can provide it to you as a part of the customization.
Related Reports:-
Cash Logistics Market Share 2023-2028
Pigments Market Growth 2023-2028
Medical Foam Market Report 2023-2028
About Us:
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARC's information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the company's expertise.
Contact Us: IMARC Services Private Limited.
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.Com Tel No:(D) +91 120 433 0800 Americas:- +1 631 791 1145Africa and Europe :- +44-702-409-7331Asia: +91-120-433-0800, +91-120-433-0800
The post Pompe Disease Treatment Market Size, Share, Business Growth & Report 2023-2028 appeared first on Super Market Research.
COMTEX_437070278/2607/2023-07-20T05:12:06
© 2023 Benzinga.Com. Benzinga does not provide investment advice. All rights reserved.



Comments
Post a Comment