Essential Thrombocythemia: Symptoms, Causes, and More
Diagnosis Of Myelodysplastic Syndrome (MDS)
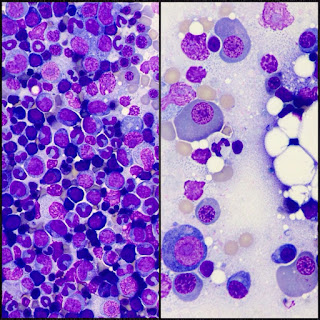
Myelodysplastic syndrome (MDS) can be difficult to diagnose.
Many people who are diagnosed with MDS go to their doctor with some or all of the symptoms typical of MDS. Other people are diagnosed with MDS even though they don't have any symptoms. They may go to their doctor for a routine checkup and mild blood-count abnormalities are found in their blood work.
Several other diseases can be confused with MDS, including:
Proper diagnosis is crucial so that you receive the most-effective treatment for MDS. Often, you will be referred to a hematologist (a doctor who specializes in blood disorders). A definitive diagnosis can be provided only after a specialist called a hematopathologist examines a sample of your bone marrow.
Blood Tests to Diagnose MDSA very basic blood test called a CBC can determine whether the numbers of various types of blood cells are within normal ranges. CBC stands for complete blood count. It is a routine test that is usually done as part of your regular medical checkup or before you have surgery.
Abnormalities in this test provide the first sign of the disease. In MDS, red blood cell levels may be low, which causes anemia. Platelet levels may also be low, which can cause bleeding and bruising. Low white blood cell levels may lead to infections.
A hematologist will also look at the blood sample under a microscope in a test called a blood smear. This can identify any abnormal cell shapes and sizes, which can also indicate MDS.
In addition, tests will be done to look for other causes of low blood counts, including thyroid disease, low vitamin levels, and iron deficiency.
Bone Marrow Tests to Diagnose MDSMyelodysplastic Syndrome (MDS) Doctors & Other Experts
Select from the list below to learn more about MSK MDS experts, their education, training, board certifications, current publications, and specific areas of expertise.
Learn moreIf blood tests do not show another reason for the abnormal blood counts, samples of your bone marrow will be examined to look for MDS. Two types of samples are taken. One is called a bone marrow biopsy. It is obtained by removing a small piece of the bone along with the marrow inside the bone. The second sample is called a bone marrow aspirate. It is obtained by drawing out liquid from the bone marrow space.
Many tests are performed on the bone marrow biopsy and aspirate samples. These tests can help confirm a diagnosis of MDS. For example, a test might show that the cells look abnormal. The tests can also determine the subtype of MDS and help doctors determine the most-effective treatment and prognosis.
The following tests are performed on bone marrow samples:
New diagnostic tests and procedures are emerging as a result of research performed at Memorial Sloan Kettering and at other institutions. Your doctor may ask whether you are willing to have additional blood or bone marrow samples taken for this type of research.
These tests are not necessary to make a diagnosis of MDS, and they are not required tests. However, research on samples taken from people with MDS is vital to our ongoing efforts to learn more about MDS and to develop better treatments. Talk with your Memorial Sloan Kettering doctor to learn more about research studies.
Myelodysplastic Syndromes: The Complexity Of Stem-cell Diseases
Aul, C., Giagounidis, A. & Germing, U. Epidemiological features of myelodysplastic syndromes: results from regional cancer surveys and hospital-based statistics. Int. J. Hematol. 73, 405–410 (2001).
Komrokji, R. Myelodysplastic syndromes: a view from where the sun rises and where the sun sets. Leuk. Res. 30, 1067–1068. (2006).
Matsuda, A. Et al. Difference in clinical features between Japanese and German patients with refractory anemia in myelodysplastic syndromes. Blood 106, 2633–2640 (2005).
Maserati, E. Et al. Familial myelodysplastic syndromes, monosomy 7/trisomy 8, and mutator effects. Cancer Genet. Cytogenet. 148, 155–158 (2004).
Minelli, A. Et al. Familial partial monosomy 7 and myelodysplasia: different parental origin of the monosomy 7 suggests action of a mutator gene. Cancer Genet. Cytogenet. 124, 147–151 (2001).
Bennett, J. M. Et al. Proposals for the classification of the myelodysplastic syndromes. Br. J. Haematol. 51, 189–199 (1982). This 25 year old classification scheme remains popular and widely used.
Vardiman, J. W., Harris, N. L. & Brunning, R. D. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 100, 2292–2302 (2002). The newer international classification scheme for all haematological neoplasms remains similar to the FAB scheme, with a crucial change in the number of blasts that defines AML.
Greenberg, P. Et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89, 2079–2088 (1997).
Howe, R. B., Porwit-MacDonald, A., Wanat, R., Tehranchi, R. & Hellstrom-Lindberg, E. The WHO classification of MDS does make a difference. Blood 103, 3265–3270 (2004).
Malcovati, L. Et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J. Clin. Oncol. 23, 7594–7603 (2005).
Fenaux, P. & Kelaidi, C. Treatment of the 5q- syndrome. Hematology Am. Soc. Hematol. Educ. Program 192–198 (2006).
Wang, J. C. & Dick, J. E. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 15, 494–501 (2005).
Wang, J. C. Y. Et al. In Hematopoiesis — A developmental approach (Ed. Zon, L. I.) 99–118 (Oxford University Press, 2001).
Lapidot, T. Et al. A cell initiating human acute myeloid leukemia after transplantation into SCID mice. Nature 367, 645–648 (1994).
Bonnet, D. & Dick, J. E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Med. 3, 730–737 (1997).
Hope, K. J., Jin, L. & Dick, J. E. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nature Immunol. 5, 738–743 (2004).
Juvonen, E., Aimolahti, A., Volin, L. & Ruutu, T. The prognostic value of in vitro cultures of erythroid and megakaryocyte progenitors in myelodysplastic syndromes. Leuk. Res. 23, 889–894 (1999).
Sato, T., Kim, S., Selleri, C., Young, N. S. & Maciejewski, J. P. Measurement of secondary colony formation after 5 weeks in long-term cultures in patients with myelodysplastic syndrome. Leukemia 12, 1187–1194 (1998).
Nilsson, L. Et al. Involvement and functional impairment of the CD34(+)CD38(-)Thy-1(+) hematopoietic stem cell pool in myelodysplastic syndromes with trisomy 8. Blood 100, 259–267 (2002).
Nilsson, L. Et al. Isolation and characterization of hematopoietic progenitor/stem cells in 5q-deleted myelodysplastic syndromes: evidence for involvement at the hematopoietic stem cell level. Blood 96, 2012–2021 (2000). This work shows the in vitro functional characterization of CD34+CD38−cells from patients with del(5q) syndrome, with implications for the identity of the MDS stem cell.
Asano, H. Et al. Evidence for nonclonal hematopoietic progenitor cell populations in bone marrow of patients with myelodysplastic syndromes. Blood 84, 588–594 (1994).
Claessens, Y. E. Et al.. In vitro proliferation and differentiation of erythroid progenitors from patients with myelodysplastic syndromes: evidence for Fas-dependent apoptosis. Blood 99, 1594–1601 (2002).
Campioni, D. Et al. Evidence for a role of TNF-related apoptosis-inducing ligand (TRAIL) in the anemia of myelodysplastic syndromes. Am. J. Pathol. 166, 557–563 (2005). TNF-related apoptosis probably has an important role in low-risk MDS, although this mechanism has not been validated by anti-TNF agents.
Benito, A. I. Et al. NOD/SCID mice transplanted with marrow from patients with myelodysplastic syndrome (MDS) show long-term propagation of normal but not clonal human precursors. Leuk. Res. 27, 425–436 (2003).
Thanopoulou, E. Et al. Engraftment of NOD/SCID-beta2 microglobulin null mice with multilineage neoplastic cells from patients with myelodysplastic syndrome. Blood 103, 4285–4293 (2004).
Kerbauy, D. M., Lesnikov, V., Torok-Storb, B., Bryant, E. & Deeg, H. J. Engraftment of distinct clonal MDS-derived hematopoietic precursors in NOD/SCID-beta2-microglobulin-deficient mice after intramedullary transplantation of hematopoietic and stromal cells. Blood 104, 2202–2203 (2004). References 25 and 26 describe the successful engraftment of clonal MDS cells in a more immunodeficient strain of NOD/SCID mice.
Ito, M. Et al. NOD/SCID/γ(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100, 3175–3182 (2002).
Shultz, L. D. Et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174, 6477–6489 (2005).
Buonamici, S. Et al. EVI1 induces myelodysplastic syndrome in mice. J. Clin. Invest. 114, 713–719 (2004). Although others have modelled high-risk MDS by engrafting primary cells in immunodeficient mouse strains, the mouse model described in this study mimics many of the features found in low-risk MDS.
Grisendi, S. Et al. Role of nucleophosmin in embryonic development and tumorigenesis. Nature 437, 147–153 (2005).
Lin, Y. W., Slape, C., Zhang, Z. & Aplan, P. D. NUP98-HOXD13 transgenic mice develop a highly penetrant, severe myelodysplastic syndrome that progresses to acute leukemia. Blood 106, 287–295 (2005). This transgenic mouse model comes closest to reproducing the different stages of MDS progression.
Moody, J. L. & Jirik, F. R. Compound heterozygosity for Pten and SHIP augments T-dependent humoral immune responses and cytokine production by CD(4+) T cells. Immunology 112, 404–412 (2004).
Disperati, P. Et al. Progression of myelodysplasia to acute lymphoblastic leukaemia: implications for disease biology. Leuk. Res. 30, 233–239 (2006).
Kardos, G. Et al. Refractory anemia in childhood: a retrospective analysis of 67 patients with particular reference to monosomy 7. Blood 102, 1997–2003 (2003).
Dong, F. Et al. Mutations in the gene for the granulocyte colony-stimulating-factor receptor in patients with acute myeloid leukemia preceded by severe congenital neutropenia. N. Engl. J. Med. 333, 487–493 (1995).
Taniguchi, T. & D'Andrea, A. D. Molecular pathogenesis of Fanconi anemia: recent progress. Blood 107, 4223–4233 (2006).
D'Andrea, A. D. & Grompe, M. The Fanconi anaemia/BRCA pathway. Nature Rev. Cancer 3, 23–34 (2003).
Liu, J. M. & Ellis, S. R. Ribosomes and marrow failure: coincidental association or molecular paradigm? Blood 107, 4583–4588 (2006).
Chiocchetti, A. Et al. Interactions between RPS19, mutated in Diamond-Blackfan anemia, and the PIM-1 oncoprotein. Haematologica 90, 1453–1462 (2005).
Amaravadi, R. & Thompson, C. B. The survival kinases Akt and Pim as potential pharmacological targets. J. Clin. Invest. 115, 2618–2624 (2005).
Boocock, G. R. Et al. Mutations in SBDS are associated with Shwachman-Diamond syndrome. Nature Genet. 33, 97–101 (2003).
Wessels, D. Et al. The Shwachman-Bodian-Diamond syndrome gene encodes an RNA-binding protein that localizes to the pseudopod of Dictyostelium amoebae during chemotaxis. J. Cell Sci. 119, 370–379 (2006).
Stepanovic, V., Wessels, D., Goldman, F. D., Geiger, J. & Soll, D. R. The chemotaxis defect of Shwachman-Diamond Syndrome leukocytes. Cell Motil. Cytoskeleton 57, 158–174 (2004).
Horwitz, M. Et al. Role of neutrophil elastase in bone marrow failure syndromes: molecular genetic revival of the chalone hypothesis. Curr. Opin. Hematol. 10, 49–54 (2003).
Ancliff, P. J. Et al. Two novel activating mutations in the Wiskott-Aldrich syndrome protein result in congenital neutropenia. Blood 108, 2182–2189 (2006).
Rosselli, F. Fanconi anaemia syndrome and apoptosis: state of the art. Apoptosis 3, 229–236 (1998).
Gazda, H. T. Et al. Defective ribosomal protein gene expression alters transcription, translation, apoptosis and oncogenic pathways in Diamond-Blackfan anemia. Stem Cells 24, 2034–2044 (2006).
Papadaki, H. A. & Eliopoulos, G. D. The role of apoptosis in the pathophysiology of chronic neutropenias associated with bone marrow failure. Cell Cycle 2, 447–451 (2003).
Dror, Y. & Freedman, M. H. Shwachman-Diamond syndrome marrow cells show abnormally increased apoptosis mediated through the Fas pathway. Blood 97, 3011–3016 (2001).
Carlsson, G. Et al. Kostmann syndrome: severe congenital neutropenia associated with defective expression of Bcl-2, constitutive mitochondrial release of cytochrome c, and excessive apoptosis of myeloid progenitor cells. Blood 103, 3355–3361 (2004).
Ruggero, D. Et al. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science 299, 259–262 (2003).
Betti, C. J., Villalobos, M. J., Diaz, M. O. & Vaughan, A. T. Apoptotic triggers initiate translocations within the MLL gene involving the nonhomologous end joining repair system. Cancer Res. 61, 4550–4555 (2001).
Betti, C. J., Villalobos, M. J., Diaz, M. O. & Vaughan, A. T. Apoptotic stimuli initiate MLL-AF9 translocations that are transcribed in cells capable of division. Cancer Res. 63, 1377–1381 (2003).
Josting, A. Et al. Secondary myeloid leukemia and myelodysplastic syndromes in patients treated for Hodgkin's disease: a report from the German Hodgkin's Lymphoma Study Group. J. Clin. Oncol. 21, 3440–3446 (2003).
Rivera, G. K., Pui, C. H., Santana, V. M., Pratt, C. B. & Crist, W. M. Epipodophyllotoxins in the treatment of childhood cancer. Cancer Chemother. Pharmacol. 34 (Suppl.), S89–S95 (1994).
Crump, M. Et al. Risk of acute leukemia following epirubicin-based adjuvant chemotherapy: a report from the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 21, 3066–3071 (2003).
Brown, J. R. Et al. Increasing incidence of late second malignancies after conditioning with cyclophosphamide and total-body irradiation and autologous bone marrow transplantation for non-Hodgkin's lymphoma. J. Clin. Oncol. 23, 2208–2214 (2005).
Viniou, N. Et al. Acute myeloid leukemia in a patient with ataxia-telangiectasia: a case report and review of the literature. Leukemia 15, 1668–1670 (2001).
Meyn, M. S. Ataxia-telangiectasia, cancer and the pathobiology of the ATM gene. Clin. Genet. 55, 289–304 (1999).
Meyer, S. Et al. Spectrum and significance of variants and mutations in the Fanconi anaemia group G gene in children with sporadic acute myeloid leukaemia. Br. J. Haematol. 133, 284–292 (2006).
Xie, Y. Et al. Aberrant Fanconi anaemia protein profiles in acute myeloid leukaemia cells. Br. J. Haematol. 111, 1057–1064 (2000).
Harada, H. Et al. High incidence of somatic mutations in the AML1/RUNX1 gene in myelodysplastic syndrome and low blast percentage myeloid leukemia with myelodysplasia. Blood 103, 2316–2324 (2004).
Harada, H., Harada, Y., Tanaka, H., Kimura, A. & Inaba, T. Implications of somatic mutations in the AML1 gene in radiation-associated and therapy-related myelodysplastic syndrome/acute myeloid leukemia. Blood 101, 673–680 (2003).
Karran, P., Offman, J. & Bignami, M. Human mismatch repair, drug-induced DNA damage, and secondary cancer. Biochimie 85, 1149–1160 (2003).
Offman, J. Et al. Defective DNA mismatch repair in acute myeloid leukemia/myelodysplastic syndrome after organ transplantation. Blood 104, 822–828 (2004).
Olipitz, W. Et al. Defective DNA-mismatch repair: a potential mediator of leukemogenic susceptibility in therapy-related myelodysplasia and leukemia. Genes Chromosomes Cancer 34, 243–248 (2002).
Allan, J. M. Et al. Polymorphism in glutathione S-transferase P1 is associated with susceptibility to chemotherapy-induced leukemia. Proc. Natl Acad. Sci. USA 98, 11592–11597 (2001).
Felix, C. A. Et al. Association of CYP3A4 genotype with treatment-related leukemia. Proc. Natl Acad. Sci. USA 95, 13176–13181 (1998).
Zang, D. Y., Goodwin, R. G., Loken, M. R., Bryant, E. & Deeg, H. J. Expression of tumor necrosis factor-related apoptosis-inducing ligand, Apo2L, and its receptors in myelodysplastic syndrome: effects on in vitro hemopoiesis. Blood 98, 3058–3065 (2001).
Reza, S. Et al. Biologic characteristics of 164 patients with myelodysplastic syndromes. Leuk. Lymphoma 33, 281–287 (1999).
Schmidt, M. Et al. Role of the CD95/CD95 ligand system in glucocorticoid-induced monocyte apoptosis. J. Immunol. 166, 1344–1351 (2001).
Tehranchi, R. Et al. Granulocyte colony-stimulating factor inhibits spontaneous cytochrome c release and mitochondria-dependent apoptosis of myelodysplastic syndrome hematopoietic progenitors. Blood 101, 1080–1086 (2003).
Allampallam, K. Et al. Biological significance of proliferation, apoptosis, cytokines, and monocyte/macrophage cells in bone marrow biopsies of 145 patients with myelodysplastic syndrome. Int. J. Hematol. 75, 289–297 (2002).
Tauro, S., Hepburn, M. D., Bowen, D. T. & Pippard, M. J. Assessment of stromal function, and its potential contribution to deregulation of hematopoiesis in the myelodysplastic syndromes. Haematologica 86, 1038–1045 (2001).
Tauro, S., Hepburn, M. D., Peddie, C. M., Bowen, D. T. & Pippard, M. J. Functional disturbance of marrow stromal microenvironment in the myelodysplastic syndromes. Leukemia 16, 785–790 (2002).
Kook, H. Et al. Increased cytotoxic T cells with effector phenotype in aplastic anemia and myelodysplasia. Exp. Hematol. 29, 1270–1277 (2001).
Tehranchi, R. Et al. Aberrant mitochondrial iron distribution and maturation arrest characterize early erythroid precursors in low-risk myelodysplastic syndromes. Blood 106, 247–253 (2005).
Albitar, M. Et al. Myelodysplastic syndrome is not merely 'preleukemia'. Blood 100, 791–798 (2002).
Hellstrom-Lindberg, E. Et al. Apoptosis in refractory anaemia with ringed sideroblasts is initiated at the stem cell level and associated with increased activation of caspases. Br. J. Haematol. 112, 714–726 (2001).
Boudard, D. Et al. Expression and activity of caspases 1 and 3 in myelodysplastic syndromes. Leukemia 14, 2045–2051 (2000).
Parker, J. E. Et al. The role of apoptosis, proliferation, and the Bcl-2-related proteins in the myelodysplastic syndromes and acute myeloid leukemia secondary to MDS. Blood 96, 3932–3938 (2000).
Boudard, D. Et al. Increased caspase-3 activity in refractory anemias: lack of evidence for Fas pathway implication. Leukemia 16, 2343–2345 (2002).
Claessens, Y. E. Et al. Rescue of early-stage myelodysplastic syndrome-deriving erythroid precursors by the ectopic expression of a dominant-negative form of FADD. Blood 105, 4035–4042 (2005). This study shows that the blockade of CD95 results in the correction of MDS-associated anaemia.
Shimazaki, K., Ohshima, K., Suzumiya, J., Kawasaki, C. & Kikuchi, M. Evaluation of apoptosis as a prognostic factor in myelodysplastic syndromes. Br. J. Haematol. 110, 584–590 (2000).
Shetty, V. Et al. Intramedullary apoptosis of hematopoietic cells in myelodysplastic syndrome patients can be massive: apoptotic cells recovered from high-density fraction of bone marrow aspirates. Blood 96, 1388–1392 (2000).
Williams, G. T., Smith, C. A., Spooncer, E., Dexter, T. M. & Taylor, D. R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature 343, 76–79 (1990).
Hellstrom-Lindberg, E. Et al. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: significant effects on quality of life. Br. J. Haematol. 120, 1037–1046 (2003).
Casadevall, N. Et al. Health, economic, and quality-of-life effects of erythropoietin and granulocyte colony-stimulating factor for the treatment of myelodysplastic syndromes: a randomized, controlled trial. Blood 104, 321–327 (2004).
Fontenay-Roupie, M. Et al. Ineffective erythropoiesis in myelodysplastic syndromes: correlation with Fas expression but not with lack of erythropoietin receptor signal transduction. Br. J. Haematol. 106, 464–473 (1999). First paper to show the importance of CD95 in mediating the ineffective erythropoiesis of MDS.
Hellstrom-Lindberg, E., Kanter-Lewensohn, L. & Ost, A. Morphological changes and apoptosis in bone marrow from patients with myelodysplastic syndromes treated with granulocyte-CSF and erythropoietin. Leuk. Res. 21, 415–425 (1997).
Silva, M. Et al. Erythropoietin can induce the expression of bcl-x(L) through Stat5 in erythropoietin-dependent progenitor cell lines. J. Biol. Chem. 274, 22165–22169 (1999).
Tehranchi, R. Et al. Antiapoptotic role of growth factors in the myelodysplastic syndromes: concordance between in vitro and in vivo observations. Clin. Cancer Res. 11, 6291–6299 (2005).
Invernizzi, R. Et al. Thalidomide treatment reduces apoptosis levels in bone marrow cells from patients with myelodysplastic syndromes. Leuk. Res. 29, 641–647 (2005).
Payvandi, F. Et al. Immunomodulatory drugs inhibit expression of cyclooxygenase-2 from TNF-α, IL-1β, and LPS-stimulated human PBMC in a partially IL-10-dependent manner. Cell. Immunol. 230, 81–88 (2004).
Raza, A. Anti-TNF therapies in rheumatoid arthritis, Crohn's disease, sepsis, and myelodysplastic syndromes. Microsc. Res. Tech. 50, 229–235 (2000).
Raza, A. Et al. Remicade as TNF suppressor in patients with myelodysplastic syndromes. Leuk. Lymphoma 45, 2099–2104 (2004).
List, A. Et al. Efficacy of lenalidomide in myelodysplastic syndromes. N. Engl. J. Med. 352, 549–557 (2005). This paper reports the definitive clinical trial that led to the FDA approval of lenalidomide for MDS.
List, A. Et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N. Engl. J. Med. 355, 1456–1465 (2006).
Yoo, C. B. & Jones, P. A. Epigenetic therapy of cancer: past, present and future. Nature Rev. Drug Discov. 5, 37–50 (2006).
Silverman, L. R. DNA methyltransferase inhibitors in myelodysplastic syndrome. Best Pract. Res. Clin. Haematol. 17, 585–594 (2004).
List, A. F., Vardiman, J., Issa, J. P. & DeWitte, T. M. Myelodysplastic syndromes. Hematology Am. Soc. Hematol. Educ. Program 297–317 (2004).
Silverman, L. R. Et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J. Clin. Oncol. 20, 2429–2440 (2002).
Kantarjian, H. Et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 106, 1794–803 (2006). References 102 and 103 comprise the definitive clinical trials that led to the FDA approval of two hypomethylating agents for MDS.
Kaminskas, E. Et al. Approval summary: azacitidine for treatment of myelodysplastic syndrome subtypes. Clin. Cancer Res. 11, 3604–3608 (2005).
Kantarjian, H. Et al. Results of a randomized study of three schedules of low-dose decitabine in higher risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood 109, 52–57 (2007).
Kuendgen, A. Et al. The histone deacetylase (HDAC) inhibitor valproic acid as monotherapy or in combination with all-trans retinoic acid in patients with acute myeloid leukemia. Cancer 106, 112–119 (2006).
Garcia-Manero, G. Et al. Phase I/II study of the combination of 5-aza-2′-deoxycytidine with valproic acid in patients with leukemia. Blood 108, 3271–3279 (2006).
Gore, S. D. Et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 66, 6361–6369 (2006).
Wang, H., Chuhjo, T., Yasue, S., Omine, M. & Nakao, S. Clinical significance of a minor population of paroxysmal nocturnal hemoglobinuria-type cells in bone marrow failure syndrome. Blood 100, 3897–3902 (2002).
Chen, G. Et al. Distinctive gene expression profiles of CD34 cells from patients with myelodysplastic syndrome characterized by specific chromosomal abnormalities. Blood 104, 4210–4218 (2004).
Rosenfeld, C. & List, A. A hypothesis for the pathogenesis of myelodysplastic syndromes: implications for new therapies. Leukemia 14, 2–8 (2000).
Greenberg, P. L. Apoptosis and its role in the myelodysplastic syndromes: implications for disease natural history and treatment. Leuk. Res. 22, 1123–1136 (1998).
Greenberg, P. L., Young, N. S. & Gattermann, N. Myelodysplastic syndromes. Hematology Am. Soc. Hematol. Educ. Program 136–161 (2002).
Liesveld, J. L., Jordan, C. T. & Phillips, G. L., II . The hematopoietic stem cell in myelodysplasia. Stem Cells 22, 590–599 (2004).
General Hospital's John J. York Gets Candid About Blood And Bone Marrow Disorder Diagnoses
General Hospital star John J. York opened up about being diagnosed with a blood and bone marrow disorder — and having to take a break on the show he has appeared on since 1991.
"I said I was going to give you an update on the reason I'm taking a little hiatus from General Hospital and here it is," York, 64 — who portrays Malcolm Scorpio on the daytime soap opera — shared in a video posted to X (formerly Twitter) on Wednesday, September 13. "So last December 2022, I was diagnosed with myelodysplastic syndrome, or MDS, and multiple smoldering myeloma — two blood and bone marrow disorders."
According to the National Cancer Institute, myelodysplastic syndromes are a group of cancers that occur when immature blood cells in the bone marrow do not become healthy blood cells. Multiple smoldering myeloma is a precancerous condition that alters certain proteins in blood and can increase plasma cells in bone marrow, per the outlet.
"Over the past many months, I've had three bone marrow biopsies, many chemo treatments — I have another one coming up in a couple of weeks — and I'm closing in on a blood stem cell transplant," the actor continued. "I've been working with some wonderful people at Be the Match in order to find a potential donor on their registry. … I just want to say thanks for all the support over the years. This isn't goodbye, this is just so long. You know I'll have to take a break for at least three, maybe four months, but I'll be back."
Trista Sutter, Selena Gomez, Ellen DeGeneres and more stars have opened up about their unexpected illnesses and injuries — read more
On Friday, September 22, York revealed that he has received some promising news in regards to finding a donor.
"I believe they found a match, a perfect match, which will make me cry," he shared on Good Morning America. "So we're going to start our testing and all that kind of stuff coming up here, in about a week. I believe the transplant will happen in November."
John J. York and Josh Kelly on 'General Hospital.' ABC/Christine BartolucciYork got emotional when talking about what he would say to the potential donor.
"Thank you for saving my life. For letting me spend more time with my wife, my daughter, my son-in-law, my grandchildren, seeing this beautiful blue sky," he explained.
News of York's potential donor comes shortly after his General Hospital costar Billy Miller — who appeared as Jason Morgan and Drew Cain on the daytime soap — died by suicide at on Friday, September 15. He was 43. A rep confirmed to Us Weekly that Miller had been struggling with manic depression at the time of his death. Miller's mother, Patricia Miller, shared that her son had battled bipolar depression for years.
Demi Lovato, Catherine Zeta-Jones, Jon Hamm, Carrie Fisher, and Ashley Judd are among the many celebrities who've admitted to struggling with depression, anxiety, or bipolar disorder
Following the tragic news, General Hospital star Maurice Benard — who plays Sonny Corinthos on the show — opened up about mental health in an important message to fans.
"Since the pandemic, I have been speaking out on suicide quite a lot," Bernard, 60, shared via Instagram on Tuesday, September 19. "In the beginning, I was hesitant. I didn't know how people would handle it. But it's been very encouraging so I have not stopped talking about it! It's really hard to understand mental illness unless you have experienced the depths of pain that comes with it."
Thank You!You have successfully subscribed.
Bernard — who detailed his journey with bipolar disorder in his 2020 memoir, Nothing General About It — continued: "If you know anyone who is going through any kind of darkness, chaos, pain … Just know, all you can do is give them love, patience, understanding, and hopefully professional help if they agree." He concluded the post with a message to anyone suffering:
"If you're feeling in any kind of way, like life is not worth living for, don't think twice to talk to someone, take it from the most fragile person in the world you can get through it," he wrote. "And life has a way of rewarding you."



Comments
Post a Comment