Robert Zakar gives back to community
Global Hemostasis Testing Systems Market Value Is Poised For Remarkable Growth, Surging US$ 3,666 Million By 2033FMI
Hemostasis Testing Systems MarketThe global hemostasis testing systems market value is likely to jump from US$ 2,149.3 million in 2023 to US$ 3,666 million by 2033. This is expected to be driven by a steady CAGR of 5.5% in the hemostasis testing systems market over the next decade.
The market is driven by the increasing advancements in the product to report accurate results for the very first time. In terms of technology, regions like North America and Europe have early access to advanced technologies. For instance, Diagnostica Stago decided to launch the Max Generation analyzers in the United States market in 2023.
To Remain Ahead of Your Competitors, Request For A Sample! Https://www.Futuremarketinsights.Com/reports/sample/rep-gb-1331
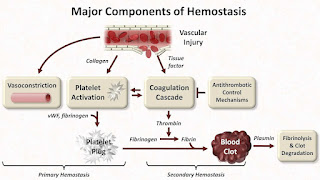
According to Stago, the pre-analytical module ascertains pre-analytical sample integrity, with checks for hemolysis, proper fill volumes, lipemia, and icterus, before testing sans the need for extra reagents, cuvettes, or plasma. These hemostasis systems empower laboratorians to report precise results with certainty. These results can then be used for immunoturbidimetric, clot-based, and colorimetric test methodologies.
Increasing FDA approvals for new hemostasis systems is expected to advance the market growth. Leading players are developing next-gen systems to enhance diagnostic capabilities. These new inventions cover the wide range of indications of the POC hemostasis analyzer.
"Key players are focusing on procuring FDA approvals for new hemostasis systems. By zeroing in on developing advanced products and bolstering the diagnostic capabilities of hemostasis testing systems, providers of hemostasis testing systems are expected to gain greater revenue from this market," says an FMI analyst.
Key Takeaways from the Hemostasis Testing Systems Market Report:
Get In Touch With Our Analyst To Resolve Any Doubts You May Have! Https://www.Futuremarketinsights.Com/ask-question/rep-gb-1331
Competitive Landscape:
Key firms can be seen leveraging cutting-edge technologies for gene, cell, and protein analysis. In tandem with this, players can be seen constantly developing their service and product portfolio, with AI-backed applications and digital offerings. Companies are providing a diverse range of services and solutions to improve the healthcare providers' capacity to offer high-quality, effective care to patients.
Key Developments Taking Place in the Hemostasis Testing Systems Market:
Key Players in the Hemostasis Testing Systems Industry:
Art of Personalization: Dive into the World of Customization with Our Report! Https://www.Futuremarketinsights.Com/customization-available/rep-gb-1331
Key Segments Profiled in the Global Hemostasis Testing Systems Market Report:
By Application Type:
By End Use:
By Region:
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 5000 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Nandini Singh Sawlani
Future Market Insights Inc.Christiana Corporate, 200 Continental Drive,Suite 401, Newark, Delaware – 19713, USAT: +1-845-579-5705For Sales Enquiries: sales@futuremarketinsights.ComWebsite: https://www.Futuremarketinsights.ComLinkedInTwitterBlogsYouTube
GLOSSARY OF HEMOSTASIS TERMS WITH ABBREVIATIONS
b-thromboglobulin bTG 11-dehydrothromboxane B2 11-DOH Activated partial thromboplastin time APTT, PTT Activated protein C APC Activated protein C resistance APCR Acute myocardial infarction AMI Adenosine diphosphate ADP Adenosine triphosphateAnti-cardiolipin antibody
Anti-phospholipid antibody
ATPACA
APL
Aspirin ASA Atrial fibrillation AFIB Bernard-Soulier syndrome BSS Cerebrovascular accident CVA Coronary artery bypass graft CABG D-dimer D-D Deep venous thrombosis DVT Dilute Russell viper venom time DRVVT Direct thrombin inhibitor DTI Disseminated intravascular coagulation DIC Ecarin clotting time ECT Endothelial cell EC Factor V Leiden mutation FVL Fibrinogen Fg Fibrin (ogen) degradation products FDP Fibrin (ogen) split products FSP Fibroblast FB Fresh frozen plasma FFP Glanzmann thrombasthenia GT Glycoprotein IIb GPIIb Heparin-induced thrombocytopenia with thrombosis HIT, HITT High molecular weight kininogen (Fitzgerald factor) HMWK Homocysteine HCY Hormone replacement therapy HRT Human platelet antigen HPA Immune (idiopathic) thrombocytopenic purpura ITP International normalized ratio INR International reference preparation IRP International sensitivity index ISI Interleukin IL Intracranial hemorrhage ICH Intramuscular IM Intravascular IV Lupus anticoagulant LA, LAC Low molecular weight heparin LMWH Neonatal alloimmune thrombocytopenic purpura NAIT Normal plasma NP Oral anticoagulant therapy OAT Oral contraceptive OCR Percutaneous coronary intervention PCI Peripheral artery occlusion PAO Platelet PLT Platelet factor 4 PF4 Platelet-free plasma PFP Platelet function analyzer PFA Platelet-poor plasma PPP Plasmin-antiplasmin PAP Plasminogen activator inhibitor-1 PAI-1 Pooled normal plasma PNP Post-transfusion purpura PTP Prekallikrein (Fletcher factor) PK Prostaglandin PG Protein C PC Protein S PS Proteins in vitamin K antagonism PIVKA Prothrombin complex concentrate PCC Prothrombin fragment 1+2 PF 1+2 Prothrombin time (protime) PT Prothrombin time ratio PTR Pulmonary embolism PE Red blood cell RBC Russell viper venom time RVVT Smooth muscle cell SMC Serine protease inhibitor SERPIN Solid-phase red blood cell agglutination assay SPRCA Staphylokinase SAK Streptokinase SK Subcutaneous SC, SQ Thrombin activated fibrinolysis inhibitor TAFI Thrombin-antithrombin TAT Thrombin clotting time TCT, TT Thrombotic thrombocytopenic purpura TTP Thrombomodulin TM Thromboxane A2 TXA2 Thromboxane B2 TXB2 Tissue factor TF Tissue plasminogen activator TPA Transient ischemic attack TIA Tumor necrosis factor TNF Twice a day BID Unfractionated (standard) heparin UFH Unstable angina UA Urokinase UK Venous thromboembolism VTE von Willebrand disease vWD von Willebrand factor vWF White blood cell WBCHEMOSTASIS BLOOD SPECIMEN MANAGEMENT
Specimen QualityThe accuracy of hemostasis laboratory tests depends on the quality of the specimen submitted. Specimens must be properly collected, labeled, stored, packaged, and transported to the laboratory. These instructions, when properly followed, ensure specimen integrity.
Preparing to Collect a Specimen
The specimen container must be properly identified with the patient's full name, medical record number, date and time of collection; and collector's initials. A properly completed request form, including a brief patient history, must accompany each specimen. Anticoagulant therapy such as heparin or Coumadin (warfarin) affects many test results and must be noted on the test request form. The ordering physician must sign the form.
Hemostasis Blood Specimen Collection
1. Draw blood into a plastic blue top tube, which contains 3.2% (0.109 M) sodium citrate anticoagulant. Allow the tube to fill to the proper level, determined by the vacuum in the tube. Most tubes contain contain 0.3 mL anticoagulant and draw 2.7 mL of blood. A "short draw" yields grossly inaccurate results. When collecting with a butterfly unit, collect a discard tube first as the tubing delivers 0.5 mL of air.
2. Immediately after collection, gently invert 6 times to mix. Do not shake. Transport to the laboratory immediately at room temperature. Do not transport on ice.
3. Centrifuge the capped tube at 2500 xg for 10 minutes. Remember: "g" stands for g-force or relative centrifugal force (RCF), not RPM. RCF is used to calculate the correct RPM for individual centrifuges.
4. Transfer plasma with a plastic pipette into a plastic centrifuge tube, cap and centrifuge an additional 10 minutes at 2500 xg to obtain platelet poor plasma, that is, plasma with a platelet count less than 10,000/uL. Specimens that are to be frozen and specimens for lupus anticoagulant and protein S activity testing must be platelet poor.
5. Using care not to disturb the button at the bottom, transfer the plasma into another clean plastic tube using a plastic pipette. Seal the tube and label with patient name, identification number, and date and time of collection.
6. The specimen must be tested within 4 hours of collection. If the assay cannot be completed within that time, it must be frozen at -70°C (step 7).
7. Freeze the specimen immediately at -70°C. Specimens should not be frozen in an ordinary household freezer nor stored in a self-defrosting freezer as the continuous freeze-thaw cycle adversely affects specimen integrity.
8. Send frozen specimens via overnight delivery on a minimum of 5 lbs. Of dry ice. Specimens must remain frozen during transport.
Hematocrit Adjustment for Hemostasis Specimen
The ratio of whole blood to anticoagulant must be 9:1 for coagulation testing. If previous testing has revealed that the patient's hematocrit exceeds 55%, the amount of anticoagulant used should be adjusted according to the following formula:
C=1.85 x 10-3 x (100-HCT) x VWhere:C= volume of 3.2% sodium citrate in mLHCT= hematocrit in percentV= volume of whole blood in mL No adjustment is necessary for a low hematocrit.© Copyright by the University of Alabama at Birmingham



Comments
Post a Comment