Hemophilia B: Definition, Symptoms, Treatment, and More
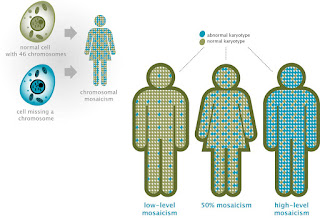
Mechanisms And Consequences Of Somatic Mosaicism In Humans
Gottlieb, B., Beitel, L. K. & Trifiro, M. A. Somatic mosaicism and variable expressivity. Trends Genet. 17, 79–82 (2001).
Wallace, D. C. & Lott, M. In Principles and Practice of Medical Genetics 4th Edn (eds Rimoin, D. L., Connor, J. M., Pyeritz, R. E. & Korf, B. R.) 299–409 (Churchill Livingstone, Edinburgh, 2002).
Hall, J. G. Twinning: mechanisms and genetic implications. Curr. Opin. Genet. Dev. 6, 343–347 (1996).
Boveri, T. The Origin of Malignant Tumors (Williams & Wilkins, Baltimore, Maryland, 1929).A timeless classic on somatic mutations and the pathogenesis of cancer.
Jackson, A. L. & Loeb, L. A. The mutation rate and cancer. Genetics 148, 1483–1490 (1998).
Lengauer, C., Kinzler, K. W. & Vogelstein, B. Genetic instabilities in human cancers. Nature 396, 643–649 (1998).
Hall, J. G. Review and hypotheses: somatic mosaicism: observations related to clinical genetics. Am. J. Hum. Genet. 43, 355–363 (1988).An excellent synthesis of observations from clinical genetics that relate to germinal and somatic mosaicism.
Youssoufian, H. Natural gene therapy and the Darwinian legacy. Nature Genet. 13, 255–256 (1996).
Hall, J. G. & Byers, P. H. Genetics of tuberous sclerosis. Lancet 1, 751 (1987).
Van Dijk, B. A., Boomsma, D. I. & De Man, A. J. M. Blood group chimerism in human multiple births is not rare. Am. J. Med. Genet. 61, 264–268 (1996).
Bianchi, D. W. & Lo, Y. M. Fetomaternal cellular and plasma DNA trafficking: the Yin and the Yang. Ann. NY Acad. Sci. 945, 119–131 (2001).
Quaini, F. Et al. Chimersim of the transplanted heart. N. Engl. J. Med. 346, 5–15 (2002).
Spangrude, G. J., Torok-Storb, B. & Little, M.-T. Chimerism of the transplanted heart. N. Engl. J. Med. 346, 1410–1412 (2002).
Bianchi, D. W., Johnson, K. L. & Salem, D. Chimerism of the transplanted heart. N. Engl. J. Med. 346, 1410–1411 (2002).
Glaser, R., Lu, M. M., Narula, N. & Epstein, J. A. Smooth muscle cells, but not myocytes, of host origin in transplanted hearts. Circulation 106, 17–19 (2002).
Ellis, N. A. Et al. Somatic intragenic recombination within the mutated locus BLM can correct the high sister-chromatid exchange phenotype of Bloom syndrome cells. Am. J. Hum. Genet. 57, 1019–1027 (1995).A landmark study on the mechanisms of somatic reversion that facilitated the cloning of the gene that is defective in Bloom syndrome.
Kvittingen, E. A., Rootwelt, H., Berger, R. & Brandtzaeg, P. Self-induced correction of the genetic defect in tyrosinemia type I. J. Clin. Invest. 94, 1657–1661 (1994).
Arredondo-Vega, F. X. Et al. Adenosine deaminase deficiency with mosaicism for a 'second-site suppressor' of a splicing mutation: decline in revertant T lymphocytes during enzyme replacement therapy. Blood 99, 1005–1013 (2002).
Gregory, J. J. Et al. Somatic mosaicism in Fanconi anemia: evidence of genotypic reversion in lymphohematopoietic stem cells. Proc. Natl Acad. Sci. USA 98, 2532–2537 (2001).
Kazazian, H. H. Retrotransposon insertions in germ cells and somatic cells. Dev. Biol. 106, 307–313 (2001).
Whitelaw, E. & Martin, D. I. K. Retrotransposons as epigenetic mediators of phenotypic variation in mammals. Nature Genet. 27, 361–365 (2001).
Poudrier, J., Lettre, F., Scriver, C. R., Larochelle, J. & Tanguay, R. M. Different clinical forms of hereditary tyrosinemia (type I) in patients with identical genotypes. Mol. Genet. Metab. 64, 119–125 (1998).
Kvittingen, E. A., Rootwelt, H., Brandtzaeg, P., Bergan, A. & Berger, R. Hereditary tyrosinemia type I. Self-induced correction of the fumarylacetoacetase defect. J. Clin. Invest. 91, 1816–1821 (1993).One of the earliest and most complete descriptions of molecular reversion of an inherited mutation.
Yang, T. P. Et al. Spontaneous reversion of novel Lesch–Nyhan mutation by HPRT gene rearrangement. Somat. Cell Mol. Genet. 14, 293–303 (1988).
Has, C. Et al. The Conradi–Hunermann–Happle syndrome (CDPX2) and emopamil binding protein: novel mutations, and somatic and gonadal mosaicism. Hum. Mol. Genet. 9, 1951–1955 (2000).
Ferguson, H. L. Et al. Mosaicism in pseudoachondroplasia. Am. J. Med. Genet. 70, 287–291 (1997).
Montgomery, R. A. Et al. Multiple molecular mechanisms underlying subdiagnostic variants of Marfan syndrome. Am. J. Hum. Genet. 63, 1703–1711 (1998).
Burrow, K. L. Et al. Dystrophin expression and somatic reversion in prednisone-treated and untreated Duchenne dystrophy. Neurology 41, 661–668 (1991).
Smith, T. A. Et al. Identification and quantification of somatic mosaicism for a point mutation in a Duchenne muscular dystrophy family. J. Med. Genet. 36, 313–315 (1999).
Costa, J.-M. Et al. Somatic mosaicism and compound heterozygosity in female hemophilia B. Blood 96, 1585–1587 (2000).
Leuer, M. Et al. Somatic mosaicism in hemophilia A: a fairly common event. Am. J. Hum. Genet. 69, 75–87 (2001).
Ainsworth, P. J. Et al. Example of somatic mosaicism in a series of de novo neurofibromatosis type 1 cases due to a maternally derived deletion. Hum. Mutat. 9, 452–457 (1997).
Sippel, K. C. Et al. Frequency of somatic and germline mosaicism in retinoblastoma: implications for genetic counseling. Am. J. Hum. Genet. 62, 610–619 (1998).
Evans, D. G. R. Et al. Somatic mosaicism: a common cause of classic disease in tumor-prone syndromes? Lessons from type 2 neurofibromatosis. Am. J. Hum. Genet. 63, 727–736 (1998).
Verhoef, S. Et al. High rate of mosaicism in tuberous sclerosis complex. Am. J. Hum. Genet. 64, 1632–1637 (1999).
Murgia, A. Et al. Somatic mosaicism in von Hippel–Lindau disease. Hum. Mutat. 15, 114 (2000).
German, J. & Ellis, N. A. In The Metabolic and Molecular Bases of Inherited Disease 8th Edn (eds Scriver, C., Beaudet, A. L., Sly, W. S. & Valle, D.) 733–752 (McGraw–Hill, New York, 2001).
Ellis, N. A. Et al. The Bloom's syndrome gene product is homologous to RecQ helicases. Cell 83, 655–666 (1995).
German, J., Ellis, N. A. & Proytcheva, M. Bloom's syndrome. XIX. Cytogenetic and population evidence for genetic heterogeneity. Clin. Genet. 49, 223–231 (1996).
Ellis, N. A., Ciocci, S. & German, J. Back mutation can produce phenotype reversion in Bloom syndrome somatic cells. Hum. Genet. 108, 167–173 (2001).
Auerbach, A. D., Buchwald, M. & Joenje, H. In Genetic Basis of Human Cancer (eds Vogelstein, B. & Kinzler, K. W.) 317–332 (McGraw–Hill, New York, 1998).
Cumming, R. C. Et al. Redox regulation of GSTP1 by the Fanconi anemia group C protein prevents apoptosis in hematopoietic cells. Nature Med. 7, 814–820 (2001).
Joenje, H. & Patel, K. J. The emerging genetic and molecular basis of Fanconi anaemia. Nature Rev. Genet. 2, 446–457 (2001).
Kwee, M. L. Et al. Unusual response to bifunctional alkylating agents in a case of Fanconi anaemia. Hum. Genet. 64, 384–387 (1983).
Auerbach, A. D. Fanconi anemia diagnosis and the diepoxybutane (DEB) test. Exp. Hematol. 21, 731–733 (1993).
Lo Ten Foe, J. R. Et al. Somatic mosaicism in Fanconi anemia: molecular basis and clinical significance. Eur. J. Hum. Genet. 5, 137–148 (1997).
Waisfisz, Q. Et al. Spontaneous functional correction of homozygous Fanconi anaemia alleles reveals novel mechanistic basis for reverse mosaicism. Nature Genet. 22, 379–383 (1999).
Timmers, C. Et al. Positional cloning of a novel Fanconi anemia gene, FANCD2. Mol. Cell 7, 241–248 (2001).
Machin, G. A. Some causes of genotypic and phenotypic discordance in monozygotic twin pairs. Am. J. Med. Genet. 61, 216–228 (1996).
Dipple, K. M. & McCabe, R. B. Phenotypes of patients with 'simple' Mendelian disorders are complex traits: thresholds, modifiers, and systems dynamics. Am. J. Hum. Genet. 66, 1729–1735 (2000).
Vijg, J. Somatic mutations and aging: a re-evaluation. Mutat. Res. 447, 117–135 (2000).
Bernards, A. & Gusella, J. F. The importance of genetic mosaicism in human disease. N. Engl. J. Med. 331, 1447–1449 (1994).
Cavenee, W. K. Et al. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. Nature 305, 779–784 (1983).
Lasko, D., Cavenee, W. & Nordenskjold, M. Loss of constitutional heterozygosity in human cancer. Annu. Rev. Genet. 25, 281–314 (1991).
Gupta, P. K. Et al. High frequency in vivo loss of heterozygosity is primarily a consequence of mitotic recombination in normal T lymphocytes of human APRT heterozygotes. Cancer Res. 57, 1188–1193 (1997).
De Nooij-Van Dalen, A. G., Morolli, B., van der Marel, A., Lohman, P. H. & Giphart-Gassler, M. Intrinsic genetic instability of normal human lymphocytes and its implication for loss of heterozygosity. Genes Chromosom. Cancer 30, 323–335 (2001).
Tobais, E. S. & Black, D. M. In Principles and Practice of Medical Genetics 4th edn (Eds Rimoin, D. L., Connor, J. M., Pyeritz, R. E. & Korf, B. R.) 514–570 (Churchill Livingstone, Edinburgh, 2002).
Aaltonen, L. A. Et al. Clues to the pathogenesis of familial colorectal cancer. Science 260, 812–816 (1993).
Kane, M. F. Et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 57, 4749–4756 (1997).
Wong, L.-J. C., Wong, H. & Liu, A. Intergenerational transmission of pathogenic heteroplasmic mitochondrial DNA. Genet. Med. 4, 78–83 (2002).
Orstavik, R. E., Tommerup, N., Eiklid, K. & Orstavik, K. H. Non-random X chromosome inactivation in an affected twin in a monozygotic twin pair discordant for Weidemann–Beckwith syndrome. Am. J. Med. Genet. 56, 210–214 (1995).
Trejo, V., Derom, C., Vlietinck, R. & Ollier, W. X-chromosome inactivation patterns correspond with fetal–placental anatomy in monozygotic twin pairs: implications for immune relatedness and concordance for autoimmunity. Mol. Med. 1, 62–70 (1995).
Bailey, W., Popovich, B. & Jones, K. L. Monozygotic twins discordant for the Russell–Silver syndrome. Am. J. Med. Genet. 58, 101–105 (1995).
Kotzot, D. Et al. Uniparental disomy 7 in Silver–Russell syndrome and primordial growth retardation. Hum. Mol. Genet. 4, 583–587 (1995).
Steinmetz, H., Herzog, A., Huang, Y. & Hacklander, T. Discordant brain-surface anatomy in monozygotic twins. N. Engl. J. Med. 331, 952–953 (1994).
Wolf, H. M. Et al. Twin boys with major histocompatibility complex class II deficiency but inducible immune responses. N. Engl. J. Med. 332, 86–90 (1995).
Berger, R. & Jonveaux, P. Clonal chromosome abnormalities in Fanconi anemia. Hematol. Cell. Ther. 38, 291–296 (1996).
Alter, B. P. Et al. Fanconi anemia: myelodysplasia as a predictor of outcome. Cancer Genet. Cytogenet. 117, 125–131 (2000).
Wallerstein, R. Et al. Common trisomy mosaicism diagnosed in amniocytes involving chromosomes 13, 18, 20 and 21: karyotype–phenotype correlations. Prenat. Diagn. 20, 103–122 (2000).
Tomie, J. L. In Principles and Practice of Medical Genetics 4th edn (Eds Rimoin, D. L., Connor, J. M., Pyeritz, R. E. & Korf, B. R.) 1129–1182 (Churchill Livingstone, Edinburgh, 2002).
Bamforth, J. S. & Lin, C. C. DK phocomelia phenotype (von Voss–Cherstvoy syndrome) caused by somatic mosaicism for del (13q). Am. J. Med. Genet. 74, 408–411 (1997).An excellent illustration of how phenotypic information that is obtained from clinical genetics can be exploited to identify somatic mosaicism.
Schinzel, A. Tetrasomy 12p (Pallister–Killian syndrome). J. Med. Genet. 28, 122–125 (1991).
Tolmie, J. L. In Principles and Practice of Medical Genetics 4th Edn (eds Rimoin, D. L., Connor, J. M., Pyeritz, R. E. & Korf, B. R.) 1129–1183 (Churchill Livingstone, Edinburgh, 2002).
Zaragoza, M. Et al. Nondisjunction of human acrocentric chromosomes. Studies of 432 fetuses and liveborns. Hum. Genet. 94, 411–417 (1994).
Allanson, J. E. & Graham, G. E. In Principles and Practice of Medical Genetics 4th Edn (eds Rimoin, D. L., Connor, J. M., Pyeritz, R. E. & Korf, B. R.) 1184–1201 (Churchill Livingstone, Edinburgh, 2002).
Robinson, W. P. Molecular studies of chromosomal mosaicism: relative frequency of chromosome gain or loss and possible role of cell selection. Am. J. Hum. Genet. 56, 444–451 (1995).
Karadima, G. Et al. Origins of nondisjunction in trisomy 8 and trisomy 8 mosaicism. Eur. J. Hum. Genet. 6, 432–438 (1998).
Sparkes, R. Et al. The validation of a 7-locus multiplex STR test for use in forensic casework. I. Mixtures, ageing, degradation and species studies. Int. J. Legal Med. 109, 186–194 (1996).
Miyamoto, T., Weissman, I. L. & Akashi, K. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc. Natl Acad. Sci. USA 97, 7521–7526 (2000).
Arredondo-Vega, F. X. Et al. Correct splicing despite mutation of the invariant first nucleotide of a 5′ splice site: a possible basis for disparate clinical phenotypes in siblings with adenosine deaminase deficiency. Am. J. Hum. Genet. 54, 820–830 (1994).
Hirschhorn, R. Et al. Spontaneous in vivo reversion to normal of an inherited mutation in a patient with adenosine deaminase deficiency. Nature Genet. 13, 290–295 (1996).
Wada, T. Et al. Somatic mosaicism in Wiskott–Aldrich syndrome suggests in vivo reversion by a DNA slippage mechanism. Proc. Natl Acad. Sci. USA 98, 8697–8702 (2001).
Stephan, V. Et al. Atypical X-linked severe combined immunodeficiency due to possible spontaneous reversion of the genetic defect in T cells. N. Engl. J. Med. 335, 1563–1567 (1996).
Joseph, J. T. Et al. Congenital myotonic dystrophy pathology and somatic mosaicism. Neurology 49, 1457–1460 (1997).
Van der Maarel, S. M. Et al. De novo fascioscapulohumeral muscular dystrophy: frequent somatic mosaicism, sex-dependent phenotype, and the role of mitotic transchromosomal repeat interaction between chromosomes 4 and 10. Am. J. Hum. Genet. 66, 26–35 (2000).
Jonkman, M. F. Et al. Revertant mosaicism in epidermolysis bullosa caused by mitotic gene conversion. Cell 88, 543–551 (1997).
Itin, P. H., Buchner, S. A. & Happle, R. Segmental manifestations of Darier disease. What is the genetic background in type 1 and type 2 mosaic phenotypes? Dermatology 200, 254–257 (2000).
Nomura, K., Umeki, K., Hatayama, I. & Kuronuma, T. Phenotypic heterogeneity in bullous congenital ichthyosiform erythroderma: possible somatic mosaicism for keratin gene mutation in the mildly affected mother of the proband. Arch. Dermatol. 137, 1192–1195 (2001).
The International IP Consortium. Survival of male patients with incontinentia pigmenti carrying a lethal mutation can be explained by somatic mosaicism or Klinefelter syndrome. Am. J. Hum. Genet. 69, 1210–1217 (2001).This study illustrates the unmasking of lethal genetic mutations in the somatic mosaic state.
Holterhus, P. M. Et al. Clinical and molecular spectrum of somatic mosaicism in androgen insensitivity syndrome. Eur. J. Pediatr. 158, 702–706 (1999).
Liehr, T. Et al. Mosaicism for the Charcot–Marie–Tooth disease type 1A duplication suggests somatic reversion. Hum. Genet. 98, 22–28 (1996).
Klopstock, T. Et al. Markedly different course of Friedreich's ataxia in sib pairs with similar GAA repeat expansions in the frataxin gene. Acta Neuropathol. 97, 139–142 (1999).
Gleeson, G. L. Et al. Somatic and germline mosaic mutations in the doublecortin gene are associated with variable phenotypes. Am. J. Hum. Genet. 67, 574–581 (2000).
Armstrong, J., Pineda, M., Aibar, E., Gean, E. & Monros, E. Classic Rett syndrome in a boy as a result of somatic mosaicism for a MECP2 mutation. Ann. Neurol. 50, 692 (2001).
Hashida, H. Et al. Single cell analysis of CAG repeat in brains of dentatorubral–pallidoluysian atrophy (DRPLA). J. Neurol. Sci. 190, 87–93 (2001).
Kato, M. Et al. Mutation of the doublecortin gene in male patients with double cortex syndrome: somatic mosaicism detected by hair root analysis. Ann. Neurol. 50, 547–551 (2001).
Darling, T. N., Yee, C., Bauer, J. W., Hintner, H. & Yancey, K. B. Revertant mosaicism: partial correction of a germ-line mutation in COL17A1 by a frame-restoring mutation. J. Clin. Invest. 103, 1371–1377 (1999).
Scheuerle, A. E. Male cases of incontinentia pigmenti: case report and review. Am. J. Med. Genet. 77, 201–218 (1998).
Parrish, J. E., Scheuerle, A. E., Lewis, R. A., Levy, M. L. & Nelson, D. L. Selection against mutant alleles in blood leukocytes is a consistent feature in incontinentia pigmenti type 2. Hum. Mol. Genet. 5, 1777–1783 (1996).
Rudolph, D. Et al. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKγ-deficient mice. Genes Dev. 14, 854–862 (2000).
Happle, R. The McCune–Albright syndrome: a lethal gene surviving by mosaicism. Clin. Genet. 29, 321–324 (1986).
Weinstein, L. S. Et al. Activating mutations of the stimulatory G protein in the McCune–Albright syndrome. N. Engl. J. Med. 325, 1688–1695 (1991).
Aldred, M. A. & Trembath, R. C. Activating and inactivating mutations in the human GNAS1 gene. Hum. Mutat. 16, 183–189 (2000).
Bianco, P. Et al. Reproduction of human fibrous dysplasia of bone in immunocompromised mice by transplanted mosaics of normal and Gsα-mutated skeletal progenitor cells. J. Clin. Invest. 101, 1737–1744 (1998).
Cohen, M. M. Jr. Proteus syndrome: clinical evidence for somatic mosaicism and selective review. Am. J. Med. Genet. 47, 645–652 (1993).
Biesecker, L. G. The multifaceted challenges of Proteus syndrome. JAMA 285, 2240–2243 (2001).
Stern, C. Somatic crossing over and segregation in Drosophila melanogaster. Genetics 21, 625–730 (1936).
Harrison, B. J. & Carpenter. R. Somatic crossing-over in Antirrhinum majus. Heredity 38, 169–189 (1977).
Lupski, J. R., Roth, J. R. & Weinstock, G. M. Chromosomal duplications in bacteria, fruit flies, and humans. Am. J. Hum. Genet. 58, 21–27 (1996).
Van Sloun, P. P. H. Et al. Determination of spontaneous loss of heterozygosity mutation in Aprt heterozygous mice. Nucleic Acids Res. 26, 4888–4894 (1998).
Shao, C. Et al. Mitotic recombination produces the majority of the recessive fibroblast variants in heterozygous mice. Proc. Natl Acad. Sci. USA 96, 9230–9235 (1999).
Liang, L., Deng, L., Shao, C., Stambrook, P. J. & Tischfield, J. A. In vivo loss of heterozygosity in T cells of B6C3F1 Aprt+/− mice. Environ. Mol. Mutagen. 35, 150–157 (2000).
Shao, C., Stambrook, P. J. & Tischfield, J. A. Mitotic recombination is suppressed by chromosomal divergence in hybrids of distantly related mouse strains. Nature Genet. 28, 169–172 (2001).An important mechanistic study on somatic mitotic recombination and its implications for tumorigenesis.
Vrieling, H. Mitotic maneuvers in the light. Nature Genet. 28, 101–102 (2001).
Johnson, R. D. & Jasin, M. Double-strand-break-induced homologous recombination in mammalian cells. Biochem. Soc. Trans. 29, 196–201 (2001).
Luo, G. Et al. Cancer predisposition caused by elevated mitotic recombination in Bloom mice. Nature Genet. 26, 424–429 (2000).
Wu, L. & Hickson, I. D. RecQ helicases and topoisomerases: components of a conserved complex for the regulation of genetic recombination. Cell Mol. Life Sci. 58, 894–901 (2001).
De Wind, N., Dekker, M., Berns, A., Radman, M. & te Riele, H. Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer. Cell 82, 321–330 (1995).
Shen, P. & Huang, H. V. Homologous recombination in Escherichia coli: dependence on substrate length and homology. Genetics 112, 441–457 (1986).
Rayssiguier, C., Thaler, D. S. & Radman, M. The barrier to recombination between Escherichia coli and Salmonella typhimurium is disrupted in mismatch-repair mutants. Nature 342, 396–401 (1989).Important mechanistic study of recombination that revealed a role for mismatch repair in prokaryotes.
Datta, A., Hendrix, M., Lipsitch, M. & Jinks-Robertson, S. Dual role for DNA sequence identity and the mismatch repair system in the regulation of mitotic crossing-over in yeast. Proc. Natl Acad. Sci. USA 94, 9757–9762 (1997).
Chen, W. & Jinks-Robertson, S. The role of the mismatch repair machinery in regulating mitotic and meiotic recombination between diverged sequences in yeast. Genetics 151, 1299–1313 (1999).
Waldman, A. S. & Liskay, R. M. Dependence of intrachromosomal recombination in mammalian cells on uninterrupted homology. Mol. Cell. Biol. 8, 5350–5357 (1988).
Manley, K., Shirley, T. L., Flaherty, L. & Messer, A. MSH2 deficiency prevents in vivo somatic instability of the CAG repeat in Huntington disease and transgenic mice. Nature Genet. 23, 471–473 (1999).
Kovtun, I. V. & McMurray, C. T. Trinucleotide expansion in haploid germ cells by gap repair. Nature Genet. 27, 407–411 (2001).
Battaile, K. P. Et al. In vivo selection of wild-type hematopoietic stem cells in a murine model of Fanconi anemia. Blood 94, 2151–2158 (1999).
Haneline, L. S. Et al. Loss of FancC function results in decreased hematopoietic stem cell repopulating ability. Blood 94, 1–8 (1999).
Soriano, P. & Jaenisch, R. Retroviruses as probes for mammalian development: allocation of cells to the somatic and germ cell lineages. Cell 46, 19–29 (1986).
Cohn, D. H., Starman, B. J., Blumberg, B. & Byers, P. H. Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a dominant mutation in a human type I collagen gene (COL1A1). Am. J. Hum. Genet. 46, 591–601 (1990).An excellent study that combines clinical observation and molecular analysis, and that quantifies the embryonic cellular contribution to the emerging somatic-cell lineages.
Wiemels, J. L. Et al. In utero origin of t(8;21) AML1–ETO translocations in childhood acute myeloid leukemia. Blood 99, 3801–3805 (2002).
Flannery, D. B. In Human Malformations and Related Anomalies, Vol. II. Oxford Monographs on Medical Genetics (eds Stevenson, R. E., Hall, J. G. & Goodman, R. M.) 907–930 (Oxford Univ. Press, New York, 1993).
Researchers Identify The Variants Responsible For A Rare And Serious Disorder
Many disorders are caused by genetic variants; to make matters worse, the genetic origin of most disorders remains unknown. Now, in a study recently published in the Journal of Clinical Immunology, researchers have shed light on the specific variants responsible for one rare and serious disorder: "RAD50 deficiency/Nijmegen breakage syndrome-like disorder."
Together with MRE11 and NBN, RAD50 is one of three proteins that make up the "MRN complex," which detects breaks in DNA and helps to initiate DNA repair. Because each of the three proteins is encoded by a separate gene, variants in any of the three genes can lead to altered functioning of the MRN complex. However, although MRE11 and NBN gene variants are known to cause other disorders, ataxia telangiectasia-like disorder and Nijmegen breakage syndrome, respectively, the pathological effects of RAD50 gene variants have remained somewhat unclear—until now.
"When we looked at the literature, we realized that only three cases of RAD50 deficiency, which leads to symptoms similar to those of Nijmegen breakage syndrome, had been reported," explains Masatoshi Takagi, lead author of the study. "Of these three, just one was reported to have RAD50 variants, with associated bone marrow failure and immunodeficiency."
When the research team came across a patient with progressive bone marrow failure and immunodeficiency combined with Nijmegen breakage syndrome-like manifestations, they decided to perform whole-exome sequencing to see if they could identify any gene variants that might lead to the observed symptoms.
"We found two different RAD50 variants in our patient, each of which was inherited from one of her parents," states Takagi. "We then tested the functional effects of these combined variants using fibroblast cells from the patient, which we grew in the lab."
The functional experiments suggested that the patient's RAD50 variants led to a loss of function of the RAD50 protein and, thus of the MRN complex. They also resulted in slower cell replication (i.E., mitosis), as expected. Interestingly, however, these variants did not cause hypersensitivity to radiation, unlike other known RAD50 variants.
"Together, the findings from our case and the three previously reported cases suggest that RAD50 deficiency/Nijmegen breakage syndrome-like disorder is characterized by growth retardation and microcephaly, which may coexist with bone marrow failure and immunodeficiency in some patients," says senior author of the study Hirokazu Kanegane. "This disorder may therefore increase susceptibility to infectious diseases and immune-related conditions."
Given the rarity of this disorder and our lack of knowledge about its genetic causes, the findings from this case are important. A better understanding of RAD50 and its effects on immunity can lead to improved diagnosis and treatment of patients with RAD50 deficiency.
More information: Masatoshi Takagi et al, Bone Marrow Failure and Immunodeficiency Associated with Human RAD50 Variants, Journal of Clinical Immunology (2023). DOI: 10.1007/s10875-023-01591-8
Citation: Researchers identify the variants responsible for a rare and serious disorder (2023, November 15) retrieved 24 November 2023 from https://medicalxpress.Com/news/2023-11-variants-responsible-rare-disorder.Html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
Are Personality Disorders Genetic? Causes And More
The exact cause of personality disorders is unclear. However, genetics, neurological differences, and environmental factors may contribute to the development of these complex mental health conditions.
Personality disorders are a class of mental health conditions marked by certain patterns of behavior, thinking, and mood. People who have personality disorders may experience distorted perceptions of reality and unusual emotional responses, which may cause distress in multiple areas of their lives.
These disorders can significantly affect a person's social functioning, relationships, and overall well-being. Both genetic and environmental factors play a role in the development of personality disorders. However, the exact relationship between genetics and these conditions is a subject of ongoing research.
In this article, we will look at whether personality disorders are genetic, as well as the causes, risk factors, disorder types, and treatments.
Personality disorders result from a combination of genetic and environmental factors. The relationship between genetics and these conditions is complex and multifaceted.
Twin studies suggest that the heritability of borderline personality disorder accounts for around 50% of a person's risk of developing it. This means that genes play a large role in whether a person develops the condition. Twin studies have also found that the heritability rate for schizoid personality disorder is 30%.
But genetics are not the only cause of personality disorders. Instead, genetics interact with environmental factors to increase or decrease the risk. Some people who have no known family history of personality disorders still develop them, and some people who have a genetic tendency toward personality disorders never develop one.
Learn more about personality disorders.
Doctors do not know the exact cause of personality disorders, but genetics may be a factor. Health experts believe that a combination of life experiences — particularly adverse childhood experiences — contributes to personality disorder development.
According to a 2016 survey of more than 1 million adults in China, people with personality disorders are more likely to be younger, unmarried, and male and to have lower socioeconomic status. These results suggest an environmental influence on the cause of these disorders.
Men are 3–5 times more likely than women to receive a diagnosis of an antisocial personality disorder, whereas borderline and histrionic personality disorders are more common in women. This indicates that genetics may play a role.
Further research is necessary to better understand the causes of personality disorders.
Understanding the risk factors associated with personality disorders is essential for recognizing and preventing these conditions. Research suggests links between genetic, social, and environmental factors, although further studies are necessary to learn more.
Risk factors for developing personality disorders include:



Comments
Post a Comment