Israeli team develops way to find genetic flaws in fetus at 11 weeks
Bionano Announces Publication Of Largest Prospective Prenatal Study Comparing OGM To Chromosomal Microarray Analysis And Karyotyping
Bionano Genomics
Key findings from prospective evaluation of 200 prenatal samples:
Optical genome mapping (OGM) detected pathogenic structural variants (SVs) in 20.5% of samples (41/200); combination of chromosomal microarray analysis (CMA) and karyotyping (KT) detected pathogenic SVs in 19.5% of samples (39/200)
Compared to CMA and KT combined in this prospective cohort, OGM had the following performance:
For 8 copy number variants (CNVs) detected by CMA, OGM defined their location and orientation and revealed that they were 6 tandem duplications and 2 unbalanced cryptic translocations
In 4% of samples (8/200), OGM identified D4Z4 repeat contractions on the permissive 4qA haplotype that may indicate facioscapulohumeral muscular dystrophy type 1 (FSHD1)
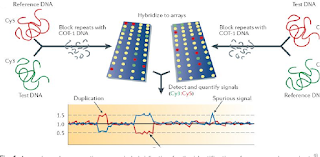
SAN DIEGO, Dec. 26, 2023 (GLOBE NEWSWIRE) -- Bionano Genomics, Inc. (Nasdaq: BNGO) today announced a publication covering the largest independent prospective prenatal study comparing optical genome mapping (OGM) to a combined workflow of chromosomal microarray analysis (CMA) and karyotyping (KT). The study highlighted OGM's high analytical concordance with the combined workflow of CMA and KT, and its ability to detect novel pathogenic structural variants (SVs) missed by those methods. The study also highlighted OGM's potential to provide additional information about SVs and copy number variants (CNVs) compared to CMA and KT.
The study, published by researchers from Nanjing Women and Children's Healthcare Hospital in China, reported that OGM demonstrated robust performance across multiple technical and analytical metrics when compared to CMA and KT. OGM also revealed the location and orientation of duplication segments, refined breakpoints of SVs and identified specific repeat contraction disorders that were not detected by other methods.
Researchers used 200 unique prospective prenatal samples with soft markers (including increased nuchal translucency, nasal bone hypoplasia and mild ventriculomegaly, and structural anomalies) for the study. Within this prospective cohort, OGM showed a 97.4% sensitivity, 100% specificity, 100% positive predictive value (PPV) and 99.4% negative predictive value (NPV) for detecting SVs reported with KT and CMA. OGM identified several additional SVs not detected by CMA and KT and defined location and orientation for eight CNVs, which may help researchers interpret the effect of CNVs more precisely. OGM also detected 8 D4Z4 repeat contractions on the permissive 4qA haplotype that may indicate facioscapulohumeral muscular dystrophy type 1 (FSHD1). The study authors concluded that OGM has the potential to serve as a first-tier cytogenetic method for prenatal analysis due to its ability to identify the majority of pathogenic SVs in a single assay.
Story continues
"The use of OGM for the analysis of prenatal samples is an area where we believe our workflow can have tremendous global impact. We believe this study marks a milestone in the Chinese prenatal research market as it includes the largest sample size to date for an independent prenatal study using OGM. Additionally, it demonstrates the workflow's strong performance and potential ability to provide answers from a single assay compared to the two to three assays required with the traditional methods commonly used today," commented Erik Holmlin, PhD, president and chief executive officer of Bionano.
The paper is available here.
About Bionano
Bionano is a provider of genome analysis solutions that can enable researchers and clinicians to reveal answers to challenging questions in biology and medicine. The Company's mission is to transform the way the world sees the genome through OGM solutions, diagnostic services and software. The Company offers OGM solutions for applications across basic, translational and clinical research. Through its Lineagen, Inc. D/b/a Bionano Laboratories business, the Company also provides diagnostic testing for patients with clinical presentations consistent with autism spectrum disorder and other neurodevelopmental disabilities. The Company also offers an industry-leading, platform-agnostic software solution, which integrates next-generation sequencing and microarray data designed to provide analysis, visualization, interpretation and reporting of copy number variants, single-nucleotide variants and absence of heterozygosity across the genome in one consolidated view. The Company additionally offers nucleic acid extraction and purification solutions using proprietary isotachophoresis technology. For more information, visit www.Bionano.Com, www.Bionanolaboratories.Com or www.Purigenbio.Com.
Unless specifically noted otherwise, Bionano's OGM products are for research use only and not for use in diagnostic procedures.
Forward-Looking Statements of Bionano Genomics
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as "believe," "can," "could," "may," and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) convey uncertainty of future events or outcomes and are intended to identify these forward-looking statements. Forward-looking statements include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things: the potential ability and utility of OGM to provide results highly concordant with CMA and KT from prenatal samples; the potential ability and utility of OGM to detect potentially pathogenic SVs in prenatal samples missed by CMA and KT; the potential ability and utility of OGM to provide information on SVs and CNVs not provided by CMA and KT; the potential ability and utility of OGM to become a first-tier test for the analysis of prenatal samples; and our ability to drive adoption of OGM and our technology solutions to be used as part of the analysis for prenatal samples. Each of these forward-looking statements involves risks and uncertainties. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include the risks and uncertainties associated with: the timing and amount of revenue we are able to recognize in a given fiscal period; the impact of adverse geopolitical and macroeconomic events, such as recent and potential future bank failures, global pandemics and the ongoing conflicts between Ukraine and Russia and Israel and Hamas, on our business and the global economy; general market conditions; changes in the competitive landscape and the introduction of competitive technologies or improvements to existing technologies; changes in our strategic and commercial plans; our ability to obtain sufficient financing to fund our strategic plans and commercialization efforts and our ability to continue as a "going concern"; the ability of medical and research institutions to obtain funding to support adoption or continued use of our technologies; study results that differ or contradict the results mentioned in this press release; failure of OGM to provide results highly concordant with CMA and KT from prenatal samples; failure of OGM to detect potentially pathogenic SVs in prenatal samples missed by CMA and KT; failure of OGM to provide information on SVs and CNVs not provided by CMA and KT; failure of OGM to become a first-tier test for the analysis of prenatal samples; failure of our ability to drive adoption of OGM and our technology solutions to be used as part of the analysis for prenatal samples; failure of and the risks and uncertainties associated with our business and financial condition in general, including the risks and uncertainties described in our filings with the Securities and Exchange Commission, including, without limitation, our Annual Report on Form 10-K for the year ended December 31, 2022 and in other filings subsequently made by us with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made and are based on management's assumptions and estimates as of such date. We do not undertake any obligation to publicly update any forward-looking statements, whether as a result of the receipt of new information, the occurrence of future events or otherwise.
CONTACTSCompany Contact:Erik Holmlin, CEOBionano Genomics, Inc.+1 (858) 888-7610eholmlin@bionano.Com
Investor Relations:David HolmesGilmartin Group+1 (858) 888-7625IR@bionano.Com
Cancers Journal Publishes Special Issue Dedicated To OGM's Impact On Hematological Malignancy Research
SAN DIEGO, Dec. 14, 2023 (GLOBE NEWSWIRE) -- Bionano Genomics, Inc. (Nasdaq: BNGO), today announced the publication of a special issue of Cancers journal entirely dedicated to the Company's optical genome mapping (OGM) workflow. The issue, which features guest editors Dr. Adam Smith from University of Toronto, Dr. Gordana Raca from Children's Hospital Los Angeles, and Dr. Alexander Hoischen from Radboud University Medical Center, includes eight peer-reviewed publications, with three additional publications expected to come, covering the use of OGM in hematological malignancy research and demonstrating its potential to offer significant research insights in a simple and effective workflow.
In an editorial, Drs. Smith, Raca and Hoischen describe the advantages OGM has over traditional cytogenetic methods of analysis, including karyotyping (KT), fluorescence in situ hybridization (FISH), and chromosomal microarray analysis (CMA), such as higher resolution and the ability to detect impactful structural variants (SVs). The guest editors also discuss limitations of long and short-read sequencing for SV detection, including challenges with resolution, sensitivity, throughput, achieved coverage, and price per genome. The guest editors note that OGM offers benefits for laboratory adoption, due to its cost-efficient and easily scalable workflow and simple analysis pipeline and software system. They conclude that OGM could enable "next generation cytogenetics" due to its potential to replace traditional cytogenetic methods and detect variants that may increase the understanding of hematologic malignancies and further expand hematological research.
The issue's other publications, authored by researchers from the United States, Canada, Germany, Finland, and a consortium of Spanish laboratories, cover research indications including acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), myeloma and myeloid cancers. These publications include findings that demonstrate OGM's high level of concordance with traditional methods for the detection of SVs, ability to identify additional pathogenic SVs missed by those methods, contributions to comprehensive tumor profiling, and potential to influence disease classification and risk prognostication.
"I personally believe the importance of OGM for hematological malignancies research cannot be overstated. What I have seen over my career as a molecular cytogeneticist is that oncologists need more information. The papers published in this dedicated issue elegantly illustrate how information provided by OGM surpasses the capabilities of the current tools in cancer research. I want to congratulate the co-editors on creating such an impactful compilation of important research results and original papers," commented Alka Chaubey, PhD, FACMG, chief medical officer of Bionano.
"This special edition of Cancers dedicated to OGM is a significant milestone for the technique and for people in the various fields of genome analysis who are looking for ways to move the community forward. We were pleased to see the guest editors and publication authors share findings that underscore OGM's potential to replace traditional cytogenetic methods and show that OGM offers significant benefits that sequencing can't offer. We agree with the editors' note that OGM is a powerful tool in the cytogenetics repertoire that may contribute to the growth and relevance of the field," stated Erik Holmlin, PhD, president and chief executive officer of Bionano.
The publication can be found here.
About BionanoBionano is a provider of genome analysis solutions that can enable researchers and clinicians to reveal answers to challenging questions in biology and medicine. The Company's mission is to transform the way the world sees the genome through OGM solutions, diagnostic services and software. The Company offers OGM solutions for applications across basic, translational and clinical research. Through its Lineagen, Inc. d/b/a Bionano Laboratories business, the Company also provides diagnostic testing for patients with clinical presentations consistent with autism spectrum disorder and other neurodevelopmental disabilities. The Company also offers an industry-leading, platform-agnostic software solution, which integrates next-generation sequencing and microarray data designed to provide analysis, visualization, interpretation and reporting of copy number variants, single-nucleotide variants and absence of heterozygosity across the genome in one consolidated view. The Company additionally offers nucleic acid extraction and purification solutions using proprietary isotachophoresis technology. For more information, visit www.Bionano.Com, www.Bionanolaboratories.Com or www.Purigenbio.Com.
Unless specifically noted otherwise, Bionano's OGM products are for research use only and not for use in diagnostic procedures.
Forward-Looking Statements of BionanoThis press release contains forward-looking statements contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as "believe," "can," "could," "may," "potential" and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances and the negatives thereof) convey uncertainty of future events or outcomes and are intended to identify these forward-looking statements. Forward-looking statements include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things: the ability and utility of OGM to detect SVs relevant to hematologic malignancies and hematological research; the potential of OGM to detect SVs compared to traditional cytogenetic techniques and long- and short-read sequencing; the potential of OGM to offer benefits for laboratory adoption; and other statements that are not historical facts. Each of these forward-looking statements involves risks and uncertainties. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include the risks and uncertainties associated with: the impact of geopolitical and macroeconomic developments, such as recent and future bank failures, the ongoing Ukraine-Russia conflict, related sanctions, the Israel-Hamas war, and any global pandemics, on our business and the global economy; challenges inherent in developing, manufacturing and commercializing products; our ability to further deploy new products and applications and expand the markets for our technology platforms; failure of OGM to detect SVs relevant to hematologic malignancies and hematological research; failure of OGM to detect SVs compared to traditional cytogenetic techniques and long- and short-read sequencing; failure of OGM to offer benefits for laboratory adoption; future publications that contradict or do not support the statements in the special issue of Cancers; our expectations and beliefs regarding future growth of the business and the markets in which we operate; changes in our strategic and commercial plans; our ability to obtain sufficient financing to fund our strategic plans and commercialization efforts and our ability to continue as a "going concern"; and including the risks and uncertainties described in our filings with the Securities and Exchange Commission, including, without limitation, our Annual Report on Form 10-K for the year ended December 31, 2022 and in other filings subsequently made by us with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made and are based on management's assumptions and estimates as of such date. We are under no duty to update any of these forward-looking statements after the date they are made to conform these statements to actual results or revised expectations, except as required by law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date the statements are made. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements contained in this press release.
CONTACTS
Company Contact:Erik Holmlin, CEOBionano Genomics, Inc.+1 (858) 888-7610eholmlin@bionano.Com
Investor Relations:David HolmesGilmartin Group+1 (858) 888-7625IR@bionano.Com
CMA Country Christmas: Here's What To Expect
Thursday, December 14, 2023
This year's "CMA Country Christmas" on ABC is the first time music greats Trisha Yearwood and Amy Grant have performed together, so they had to go big.
OTRC
This year's "CMA Country Christmas" on ABC is the first time music greats Trisha Yearwood and Amy Grant have performed together, so they had to go big.
"The show starts with high energy," Yearwood told On The Red Carpet. "We rehearsed yesterday and I can't wait for people to see the show open. When you see it, you're gonna be in the Christmas spirit. You're not gonna have a choice."
Yearwood and Grant are co-hosting the annual holiday special which airs Thursday, December 14 and streams the next day on Disney+ and Hulu.
"The opening is the song, "Joy to the World," Grant told On The Red Carpet. "And we're actually doing it with another artist named Linsey Sterling and she-her physical prowess is like a freak of nature. She plays violin while she's dancing like crazy stretching high kicks and she's playing. It's all live, I mean she is freakishly good!"
Other performers include Ashley McBryde, Jordan Davis and recently crowned CMA Entertainer of the Year, Lainey Wilson who is performing "Go Tell It on the Mountain" with Christian music star, Zach Williams.
"Christmas music makes you feel at home," Wilson told On The Red Carpet. "It's pretty similar to Country music. It tells a story... And I'm so excited that I get to do this Zach Williams. I'm a big fan of his and I love his version of this song so it's gonna be pretty fun.
Other highlights include The War and Treaty getting the crowd on its feet with a rousing rendition of "Christmas (Baby, Please Come Home)" and Jon Pardi singing ""Beer for Santa."
Meanwhile, Lady A is making their 7th appearance on CMA Country Christmas with something fans have never seen from them before!
"We've got dancers, Lady A's Dave Haywood told On The Red Carpet. "First time in Lady A history we have dancers on stage... So this is something you do not want to miss"
"CMA Country Christmas" was filmed in Nashville before a live audience. It airs Thursday, December 14 on ABC at 8/7c.
Copyright © 2023 OnTheRedCarpet.Com. All Rights Reserved.



Comments
Post a Comment