Male-to-male transmission of X-linked Alport syndrome in a boy with a 47,XXY karyotype | European Journal of Human ...
Pursuing Scientific Curiosity To Strengthen The Rare Blood Disorder Community
Your browser is not supportedusatoday.Comusatoday.Com wants to ensure the best experience for all of our readers, so we built our site to take advantage of the latest technology, making it faster and easier to use.
Unfortunately, your browser is not supported. Please download one of these browsers for the best experience on usatoday.Com
FDA Approves Merck's New Drug For A Rare Lung Disease
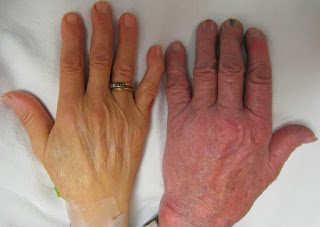
Pulmonary arterial hyptertension disproportionately impacts women of color, though its ultimate cause is still a mystery.
gettyNearly 40,000 Americans are known to suffer from pulmonary arterial hypertension (PAH), a disease that causes the blood vessels in their lungs to progressively shrink, increasing both blood pressure and the risk of heart failure. Around 500 to 1,000 new cases are diagnosed every year, with the average patient only surviving about five to seven years after being diagnosed. The vast majority of people with this disease are women, and they are disproportionately Black or Hispanic.
On Tuesday, the Food and Drug Administration approved a new drug to treat this disease called sotatercept which was developed and being marketed by pharmaceutical giant Merck under the name Winrevair. This is the first new type of treatment for the disease approved in nearly a decade, and is considered by the FDA to be "first in class," meaning it's the first medicine of its kind to treat this disease. In a clinical trial, the drug reduced the risk of death or increased severity of the disease by 83%.
According to Eliav Barr, Merck's head of global clinical development and chief medical officer, patients with this disease are highly engaged with managing it, and that there's been "enormous enthusiasm and anticipation for the drug to come out," he told Forbes. "We anticipate there's going to be very vigorous uptake."
What exactly causes PAH isn't precisely understood, Dr. Panagis Galiatsatos, a research pulmonologist at Johns Hopkins School of Medicine who was not involved in developing the drug, explained to Forbes. For some patients there appears to be a genetic component, but in others it's unknown. This variety of causes and the rarity of the disease make it a "double-edged sword" for researchers trying to develop treatments, he said. "That's tough, because you struggle to know if one treatment will help out."
In patients with PAH, the blood vessels begin to grow inwardly, leaving less and less room for blood to flow freely. This increases blood pressure in the lungs, forcing the right side of the heart to pump harder. As the disease progresses, it becomes more difficult for patients to get a lot of exercise or even perform daily tasks as it becomes harder for them to breathe. Heart failure is common.
Unlike previous drugs for this disease, which either treat symptoms or alleviate blood pressure by dilating the blood vessels, sotatercept actually attacks the signaling mechanisms in the body that have gone wrong and tell the blood vessels to keep growing, explained Barr. The drug works by blocking those signals, which causes the blood vessels to return to a normal thickness, which in turn lowers blood pressure.
In a clinical trial for the drug that was published in the New England Journal of Medicine, researchers found that sotatercept, when injected at 3-week intervals, enabled patients to extend the distance they could walk in 6 minutes by over 40 meters compared to those who received a placebo. The trial also found that the risk of death or a significant worsening of the disease progression was reduced by 84% compared to placebo. "It was just a dramatic reduction in the rate of progression and worsening of clinical symptoms," said Barr.
With the drug now approved by the FDA, it will just be a "matter of weeks" before it hits the market, Barr said. And Galiatsatos said that because of the relative dearth of treatment options for patients with PAH, he expects that sotatercept will become one of the first drugs prescribed to PAH patients "sooner rather than later."
That's a sentiment shared by analysts at investment banking firm Jefferies, which in a report published earlier this year estimated $7.5 billion in peak 2024 sales for the drug based on FDA approval and its expected European approval in the second half of the year. The firm added a possible upside of over $9 billion in sotatercept sales.
Merck's scientists also think that the drug can be useful for other types of pulmonary hypertension. The company is currently conducting a phase 2 clinical trial in a group of patients who have pulmonary hypertension as a result of a certain type of heart failure. If it's successful with that group, the company could rapidly expand the number of people who can benefit from the medication.
"It's a pretty big breakthrough," Barr said, adding that he expects the drug to help patients live longer lives. "They'll see their kids growing up and they'll be able to live better lives."
MORE AT FORBES MORE FROM FORBESThe FDA Approved A New Medication To Prevent A Common Hospital InfectionBy Alex KnappMORE FROM FORBESAbridge Raises $150 Million To Make AI Medical Scribes Even SmarterBy Katie JenningsMORE FROM FORBESAbbVie's New 'Biological Missile' Ovarian Cancer Treatment Gets Full FDA ApprovalBy James FarrellMORE FROM FORBESThis Startup Is One Step Closer To Making Drugs In SpaceBy Alex KnappRocket Pharmaceuticals Shines Spotlight On Rare Diseases
Patient at Rocket Pharmaceuticals Rare Disease day.
Mark Jaworski StudiosRocket Pharmaceuticals celebrated its sixth annual Rare Disease Day event bringing together leaders in the biotech community along with patients, patient advocates and Rocket employees. Every year the event highlights the progress Rocket is making towards finding gene therapy cures for rare and devastating diseases while offering hope to patient communities. Approximately 400 million people worldwide and 30 million people in the US live with a rare disease.
Rare diseases impact more people and families than all forms of cancer and AIDS combined and nine million children living with a rare disease pass away before their fifth birthday every year. Other rare diseases include Cystic Fibrosis, Hemophilia, Duchenne Muscular Dystrophy (DMD), and Sickle Cell and Ehlers-Danlos Syndrome (EDS), to name a few. It takes an average of six to eight years for patients to receive an accurate diagnosis of a rare disease. Symptoms vary not only between diseases but also among patients with the same condition. Approximately 72% of rare diseases are genetic, and only about 5% have an FDA-approved drug treatment.
DCM (Dilated Cardiomyopathy) patient "Becky" took to the stage during the daylong event at the Liberty Science Center to share how her rare disease has affected her life and how she's found support from her community, "This is a chronic illness. There is no cure. I'm on tons of medication. I suffer from fatigue and brain fog, low blood pressure and some other unpleasant side effects. I think it's made harder because I don't necessarily present as sick to the outside world, so it makes it challenging to ask for help" She added,"I joined the DCM support groups and it's crazy how these people from all across the globe, all genders, ages, races...All walks of life, have quickly become my friends and my confidantes."
Maneet Ahuja, Rocket President & COO Kinnari Patel and Rocket CEO Gaurav Shah, M.D.
Mark Jaworski StudiosIn a panel moderated by Forbes Editor-At-Large, Maneet Ahuja, Rocket CEO Dr. Gaurav Shah explained how gene therapy can potentially cure genetic-based diseases. "Gene therapy is one of the rare modalities in medicine that has emerged in the history of our species that is truly potentially curative because you're replacing the core DNA that's the basis of who we are as physical beings, you're replacing faulty DNA with correct DNA so there's no better cure to genetic diseases than gene therapy," he shared. "We've cracked the door open. Our goal is to push the door open all the way."
Rocket sheds light on rare diseases.
Mark Jaworski StudiosRocket President and COO Kinnari Patel also suggested getting genetically tested sooner rather than later if you are having trouble with receiving a diagnosis. "I recommend everyone get tested. Don't wait for the symptoms to appear, for you to feel uncomfortable, or having to go to the fourth or the fifth hospital to figure out what's wrong with you. The beauty of genetic testing now versus twenty years ago when it cost ten or twenty thousand dollars, it now costs a few hundred dollars, if not less, and most insurance companies cover it."
Rocket's long-term vision is utilizing gene therapy to cure genetically modulated diseases, starting with diseases with single gene defects. Rare diseases may be rare individually, but collectively they are not rare. They also hope to unlock the ability to pursue cures for polygenic diseases as well. Currently, Rocket is working on gene therapies for LAD-I, FA, PKD, Danon, PKP2-ACM, and BAG3-DCM.



Comments
Post a Comment