A familial case of MYH9 gene mutation associated with multiple functional and structural platelet abnormalities ...
Biological Dosimetry: Chromosomal Aberration Analysis For Dose Assessment
Cite this content as:INTERNATIONAL ATOMIC ENERGY AGENCY, Biological Dosimetry: Chromosomal Aberration Analysis for Dose Assessment, Technical Reports Series No. 260, IAEA, Vienna (1986)
Download to:EndNote BibTeX*use BibTeX for Zotero
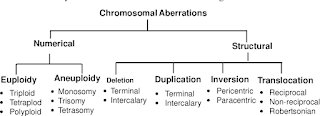
Chromosomal Aberrations Play A Major Role In Autism
A study, resulting from genome-wide scans of families affected with by autism spectrum disorder (ASD), has shown that previously unknown chromosomal aberrations have an important role to play in the ubiquitous disorder. The researchers have said that structural variations in chromosomes influence ASD and they suggest a routine clinical workup. This report is published online on the 17th January in the American Journal of Human Genetics, a publication of Cell Press. "Historical studies in identical twins and their families have provided strong evidence for a genetic basis of autism," said Stephen Scherer of The Hospital for Sick Children and the University of Toronto. "Last year, with the Autism Genome Project Consortium, we did an initial study to look at the rate of chromosomal changes in autism. Now, we've really pinned down those numbers."Autism is a complex developmental disorder found in about one in every 165 children, making it one of the most common forms of developmental disability of childhood. Individuals with ASD have deficits in social interaction and communication and show a preference for repetitive, stereotyped activities. Structural changes, including gains and losses of genes as well as chromosomal translocations (in which a chromosomal segment ends up in the wrong place) or inversions (in which a portion of the genome is oriented backwards) have been previously identified in some individuals with ASD, but their causal role hasn't been clear.
In the new study, the researchers examined structural abnormalities in 427 unrelated ASD cases using both microarray analysis and karyotyping. Microarrays can detect "unbalanced" genetic changes that alter the number of copies of a particular gene. Karyotyping, in which chromosomes are viewed under the microscope, can identify "balanced" translocations or inversions that might otherwise be missed by microarrays.
While most chromosomal abnormalities were inherited, the researchers found that seven percent of children with autism carry structural changes in the genome that are not found in their parents. The rate of such de novo changes in the general population is typically less than one percent, Scherer said.
The researchers detected 13 regions of the genome with overlapping or recurrent chromosomal changes in unrelated people with autism, suggesting that genes located at these sites may cause or add to the complexity of the condition. The most prevalent change, occurring in one percent of ASD cases, was found on chromosome 16, they reported. The altered portion of chromosome 16 has structural characteristics that make it more prone to errors, Scherer noted.
In a subset of ASD cases, the researchers found abnormalities in several genes known to be involved in neuron function. They also identified at least two sites that have previously been linked to mental retardation.
Advertisement
"Our understanding of the full etiologic role of structural variation in ASD will require genomic and phenotypic analyses of more cases (and their families) and population controls," the researchers concluded. As a first step toward achieving the desired numbers, the researchers have established a new Autism Chromosome Rearrangement Database, which allows integration of their new data and all other molecular information with the wealth of karyotypic data gathered over the years.In light of the new findings, Scherer's team also calls for new testing in the clinic.
Advertisement
"From our current data it is already apparent that for a proportion of individuals, it will be possible to describe their ASD based on the underlying structural characteristics of their genome," they wrote."If we found certain changes, we could then watch those children closer," Scherer added, noting the critical importance of early diagnosis for autism.
Source-EurekalertLIN/S
The Anaplastic Lymphoma Kinase In The Pathogenesis Of Cancer
Jaffe, E. S., Harris, N. L., Stein, H. & Vardiman, J. W. World Health Organization Classification of Tumors: Tumors of the Haematopoietic and Lymphoid Tissues (International Agency for Research on Cancer, Lyon, 2001).
Stein, H. Et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed–Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood 66, 848–858 (1985).
Stein, H. Et al. CD30+ anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood 96, 3681–3695 (2000).
Sandlund, J. T. Et al. Clinical features and treatment outcome for children with CD30+ large-cell non-Hodgkin's lymphoma. J. Clin. Oncol. 12, 895–898 (1994).
Kadin, M. E. & Morris, S. W. The t(2;5) in human lymphomas. Leuk. Lymphoma 29, 249–256 (1998).
Rizvi, M. A., Evens, A. M., Tallman, M. S., Nelson, B. P. & Rosen, S. T. T-cell non-Hodgkin lymphoma. Blood 107, 1255–1264 (2006).
Savage, K. S. Peripheral T-cell lymphomas. Blood Rev. 4, 201–216 (2007).
Fischer, P. Et al. A Ki-1 (CD30)-positive human cell line (Karpas 299) established from a high-grade non-Hodgkin's lymphoma, showing a 2;5 translocation and rearrangement of the T-cell receptor β-chain gene. Blood 72, 234–240 (1988).
Benz-Lemoine, E. Et al. Malignant histiocytosis: a specific t(2;5)(p23;q35) translocation? Review of the literature. Blood 72, 1045–1047 (1988).
Mason, D. Y. Et al. CD30-positive large cell lymphomas ('Ki-1 lymphoma') are associated with a chromosomal translocation involving 5q35. Br. J. Haematol. 74, 161–168 (1990).
Morris, S. W. Et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 263, 1281–1284 (1994). This is the first paper to describe the cloning of the ALK gene in the chromosomal breakpoints that are associated with the balanced t(2;5)(p23;q35) chromosomal translocation in ALCL.
Medeiros, L. J. & Elenitoba-Johnson, K. S. Anaplastic large cell lymphoma. Am. J. Clin. Pathol. 127, 707–722 (2007).
Benharroch, D. Et al. ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood 91, 2076–2084 (1998).
Ladanyi, M. & Cavalchire, G. Molecular variant of the NPM–ALK rearrangement of Ki-1 lymphoma involving a cryptic ALK splice site. Genes Chromosomes Cancer 15, 173–177 (1996).
Chan, P. K. & Chan, F. Y. Nucleophosmin/B23 (NPM) oligomer is a major and stable entity in HeLa cells. Biochim. Biophys. Acta 1262, 37–42 (1995).
Liu, Q. R. & Chan, P. K. Formation of nucleophosmin/B23 oligomers requires both the amino- and the carboxyl-terminal domains of the protein. Eur. J. Biochem. 200, 715–721 (1991).
Bischof, D., Pulford, K., Mason, D. Y. & Morris, S. W. Role of the nucleophosmin (NPM) portion of the non-Hodgkin's lymphoma-associated NPM-anaplastic lymphoma kinase fusion protein in oncogenesis. Mol. Cell Biol. 17, 2312–2325 (1997).
Grisendi, S., Mecucci, C., Falini, B. & Pandolfi, P. P. Nucleophosmin and cancer. Nature Rev. Cancer 6, 493–505 (2006).
Hernandez, L. Et al. Diversity of genomic breakpoints in TFG-ALK translocations in anaplastic large cell lymphomas: identification of a new TFG-ALK(XL) chimeric gene with transforming activity. Am. J. Pathol. 160, 1487–1494 (2002).
Colleoni, G. W. Et al. ATIC-ALK: A novel variant ALK gene fusion in anaplastic large cell lymphoma resulting from the recurrent cryptic chromosomal inversion, inv(2)(p23q35). Am. J. Pathol. 156, 781–789 (2000).
Ma, Z. Et al. Inv(2)(p23q35) in anaplastic large-cell lymphoma induces constitutive anaplastic lymphoma kinase (ALK) tyrosine kinase activation by fusion to ATIC, an enzyme involved in purine nucleotide biosynthesis. Blood 95, 2144–2149 (2000).
Touriol, C. Et al. Further demonstration of the diversity of chromosomal changes involving 2p23 in ALK-positive lymphoma: 2 cases expressing ALK kinase fused to CLTCL (clathrin chain polypeptide-like). Blood 95, 3204–3207 (2000).
Tort, F. Et al. Molecular characterization of a new ALK translocation involving moesin (MSN–ALK) in anaplastic large cell lymphoma. Lab. Invest. 81, 419–426 (2001).
Ventura, R. A. Et al. Centrosome abnormalities in ALK-positive anaplastic large-cell lymphoma. Leukemia 18, 1910–1911 (2004).
Armstrong, F., Lamant, L., Hieblot, C., Delsol, G. & Touriol, C. TPM3–ALK expression induces changes in cytoskeleton organisation and confers higher metastatic capacities than other ALK fusion proteins. Eur. J. Cancer 43, 640–646 (2007).
Maes, B. Et al. The NPM–ALK and the ATIC–ALK fusion genes can be detected in non-neoplastic cells. Am. J. Pathol. 158, 2185–2193 (2001).
Trumper, L., Pfreundschuh, M., Bonin, F. V. & Daus, H. Detection of the t(2;5)-associated NPM/ALK fusion cDNA in peripheral blood cells of healthy individuals. Br. J. Haematol. 103, 1138–1144 (1998).
Basecke, J. Et al. Transcription of AML1/ETO in bone marrow and cord blood of individuals without acute myelogenous leukemia. Blood 100, 2267–2268 (2002).
Bose, S., Deininger, M., Gora-Tybor, J., Goldman, J. M. & Melo, J. V. The presence of typical and atypical BCR–ABL fusion genes in leukocytes of normal individuals: biologic significance and implications for the assessment of minimal residual disease. Blood 92, 3362–3367 (1998).
Graninger, W. B., Seto, M., Boutain, B., Goldman, P. & Korsmeyer, S. J. Expression of Bcl-2 and Bcl-2-Ig fusion transcripts in normal and neoplastic cells. J. Clin. Invest. 80, 1512–1515 (1987).
Braig, M. Et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436, 660–665 (2005).
Delsol, G. Et al. A new subtype of large B-cell lymphoma expressing the ALK kinase and lacking the 2;5 translocation. Blood 89, 1483–1490 (1997). First description of the presence of ALK protein in lymphomas that are different from ALCL, that is, large B-cell lymphomas.
Kuefer, M. U. Et al. Retrovirus-mediated gene transfer of NPM–ALK causes lymphoid malignancy in mice. Blood 90, 2901–2910 (1997). This paper demonstrates for the first time the direct pathogenetic role for the NPM–ALK fusion tyrosine kinase in human lymphomas by using a retroviral gene transfer mouse model.
Lange, K. Et al. Overexpression of NPM-ALK induces different types of malignant lymphomas in IL-9 transgenic mice. Oncogene 22, 517–527 (2003).
Chiarle, R. Et al. NPM–ALK transgenic mice spontaneously develop T-cell lymphomas and plasma cell tumors. Blood 101, 1919–1927 (2003). Development of the first mouse model of ALK-induced lymphomagenesis by targeted expression of human NPM–ALK in T cells.
Jager, R. Et al. Mice transgenic for NPM-ALK develop non-Hodgkin lymphomas. Anticancer Res. 25, 3191–3196 (2005).
Chiarle, R. Et al. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nature Med. 11, 623–629 (2005). This is the first genetic study that directly shows, through gene ablation, that STAT3 is required for cellular transformation and/or for tumour survival and growth of lymphoid cells expressing NPM–ALK. It also suggests the basis for therapeutic strategies directed against STAT3 in ALCL.
Turner, S. D. & Alexander, D. R. What have we learnt from mouse models of NPM-ALK-induced lymphomagenesis? Leukemia 19, 1128–1134 (2005).
Turner, S. D., Tooze, R., Maclennan, K. & Alexander, D. R. Vav-promoter regulated oncogenic fusion protein NPM-ALK in transgenic mice causes B-cell lymphomas with hyperactive Jun kinase. Oncogene 22, 7750–7761 (2003).
Dirks, W. G. Et al. Expression and functional analysis of the anaplastic lymphoma kinase (ALK) gene in tumor cell lines. Int. J. Cancer 100, 49–56 (2002).
Soda, M. Et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448, 561–566 (2007). First description of EML4 – ALK fusion gene in a subset of lung cancers. These cancers are mutually exclusive from those that harbour EGFR mutations.
Perez-Pinera, P., Chang, Y., Astudillo, A., Mortimer, J. & Deuel, T. F. Anaplastic lymphoma kinase is expressed in different subtypes of human breast cancer. Biochem. Biophys. Res. Commun. 358, 399–403 (2007).
Griffin, C. A. Et al. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 59, 2776–2780 (1999). Description of the first known fusion involving ALK in non-haematopoietic tumours.
Lawrence, B. Et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am. J. Pathol. 157, 377–384 (2000).
Coffin, C. M., Hornick, J. L. & Fletcher, C. D. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am. J. Surg. Pathol. 31, 509–520 (2007).
Ma, Z. Et al. Fusion of ALK to the Ran-binding protein 2 (RANBP2) gene in inflammatory myofibroblastic tumor. Genes Chromosomes Cancer 37, 98–105 (2003).
Debelenko, L. V. Et al. Identification of CARS–ALK fusion in primary and metastatic lesions of an inflammatory myofibroblastic tumor. Lab. Invest. 83, 1255–1265 (2003).
Lamant, L. Et al. Expression of the ALK tyrosine kinase gene in neuroblastoma. Am. J. Pathol. 156, 1711–1721 (2000).
Powers, C., Aigner, A., Stoica, G. E., McDonnell, K. & Wellstein, A. Pleiotrophin signaling through anaplastic lymphoma kinase is rate-limiting for glioblastoma growth. J. Biol. Chem. 277, 14153–14158 (2002).
Mathivet, T., Mazot, P. & Vigny, M. In contrast to agonist monoclonal antibodies, both C-terminal truncated form and full-length form of Pleiotrophin failed to activate vertebrate ALK (anaplastic lymphoma kinase)? Cell Signal 19, 2434–2443 (2007).
Stoica, G. E. Et al. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J. Biol. Chem. 277, 35990–35998 (2002).
Perez-Pinera, P., Zhang, W., Chang, Y., Vega, J. A. & Deuel, T. F. Anaplastic lymphoma kinase is activated through the pleiotrophin/receptor protein-tyrosine phosphatase β/ζ signaling pathway: an alternative mechanism of receptor tyrosine kinase activation. J. Biol. Chem. 282, 28683–28690 (2007).
Pillay, K., Govender, D. & Chetty, R. ALK protein expression in rhabdomyosarcomas. Histopathology 41, 461–467 (2002).
Jazii, F. R. Et al. Identification of squamous cell carcinoma associated proteins by proteomics and loss of β tropomyosin expression in esophageal cancer. World J. Gastroenterol. 12, 7104–7112 (2006).
Du, X. L. Et al. Proteomic profiling of proteins dysregulted in Chinese esophageal squamous cell carcinoma. J. Mol. Med. 85, 863–875 (2007).
Wellmann, A. Et al. The activated anaplastic lymphoma kinase increases cellular proliferation and oncogene up-regulation in rat 1a fibroblasts. FASEB J. 11, 965–972 (1997).
Ambrogio, C. Et al. P130Cas mediates the transforming properties of the anaplastic lymphoma kinase. Blood 106, 3907–3916 (2005).
Bai, R. Y., Dieter, P., Peschel, C., Morris, S. W. & Duyster, J. Nucleophosmin-anaplastic lymphoma kinase of large-cell anaplastic lymphoma is a constitutively active tyrosine kinase that utilizes phospholipase C-γ to mediate its mitogenicity. Mol. Cell Biol. 18, 6951–6961 (1998).
Bai, R. Y. Et al. Nucleophosmin–anaplastic lymphoma kinase associated with anaplastic large-cell lymphoma activates the phosphatidylinositol 3-kinase/Akt antiapoptotic signaling pathway. Blood 96, 4319–4327 (2000). First identification of PI3K and Akt as downstream effectors of the NPM–ALK anti-apoptotic signalling pathway and their contribution to the molecular pathogenesis of ALCL.
Zamo, A. Et al. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene 21, 1038–1047 (2002). First report showing that JAK3 and STAT3 are constitutively activated in ALK-positive cells and that activation of STAT3 contributes to the growth and resistance to apoptosis of ALK-positive tumour cells.
Raetz, E. A. Et al. The nucleophosmin-anaplastic lymphoma kinase fusion protein induces c-Myc expression in pediatric anaplastic large cell lymphomas. Am. J. Pathol. 161, 875–883 (2002).
Fujimoto, J. Et al. Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki-1 lymphoma cell line with chromosomal translocation t(2;5). Proc. Natl Acad. Sci. USA 93, 4181–4186 (1996). First demonstration of the transforming properties of NPM–ALK in vitro in mouse fibroblasts.
Pulford, K., Morris, S. W. & Turturro, F. Anaplastic lymphoma kinase proteins in growth control and cancer. J. Cell Physiol. 199, 330–358 (2004).
Voena, C. Et al. The tyrosine phosphatase Shp2 interacts with NPM-ALK and regulates anaplastic lymphoma cell growth and migration. Cancer Res. 67, 4278–4286 (2007).
Sattler, M. Et al. Critical role for Gab2 in transformation by BCR/ABL. Cancer Cell 1, 479–492 (2002).
Cussac, D. Et al. Nucleophosmin-anaplastic lymphoma kinase of anaplastic large-cell lymphoma recruits, activates, and uses pp60c-src to mediate its mitogenicity. Blood 103, 1464–1471 (2004).
Bacchiocchi, R. Et al. Activation of α-diacylglycerol kinase is critical for the mitogenic properties of anaplastic lymphoma kinase. Blood 106, 2175–2182 (2005).
Honorat, J. F., Ragab, A., Lamant, L., Delsol, G. & Ragab-Thomas, J. SHP1 tyrosine phosphatase negatively regulates NPM–ALK tyrosine kinase signaling. Blood 107, 4130–4138 (2006).
Han, Y. Et al. Restoration of shp1 expression by 5-aza-2′-deoxycytidine is associated with downregulation of JAK3/STAT3 signaling in ALK-positive anaplastic large cell lymphoma. Leukemia 20, 1602–1609 (2006).
Han, Y. Et al. Loss of SHP1 enhances JAK3/STAT3 signaling and decreases proteosome degradation of JAK3 and NPM–ALK in ALK+ anaplastic large-cell lymphoma. Blood 108, 2796–2803 (2006).
Leventaki, V. Et al. NPM–ALK oncogenic kinase promotes cell cycle progression through activation of JNK/c-Jun signaling in anaplastic large cell lymphoma. Blood 110, 1621–1630 (2007).
Ouyang, T. Et al. Identification and characterization of a nuclear interacting partner of anaplastic lymphoma kinase (NIPA). J. Biol. Chem. 278, 30028–30036 (2003).
Bassermann, F. Et al. Multisite phosphorylation of nuclear interaction partner of ALK (NIPA) at G2/M involves cyclin B1/Cdk1. J. Biol. Chem. 282, 15965–15972 (2007).
Bassermann, F. Et al. NIPA defines an SCF-type mammalian E3 ligase that regulates mitotic entry. Cell 122, 45–57 (2005).
Gu, T. L. Et al. NPM-ALK fusion kinase of anaplastic large-cell lymphoma regulates survival and proliferative signaling through modulation of FOXO3a. Blood 103, 4622–4629 (2004).
Zhang, Q. Et al. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma. J. Immunol. 168, 466–474 (2002).
Amin, H. M. Et al. Inhibition of JAK3 induces apoptosis and decreases anaplastic lymphoma kinase activity in anaplastic large cell lymphoma. Oncogene 22, 5399–5407 (2003).
Marzec, M. Et al. Inhibition of ALK enzymatic activity in T-cell lymphoma cells induces apoptosis and suppresses proliferation and STAT3 phosphorylation independently of Jak3. Lab. Invest. 85, 1544–1554 (2005).
Coluccia, A. M. Et al. Bcl-XL down-regulation suppresses the tumorigenic potential of NPM/ALK in vitro and in vivo. Blood 103, 2787–2794 (2004).
Piva, R. Et al. Functional validation of the anaplastic lymphoma kinase signature identifies CEBPB and BCL2A1 as critical target genes. J. Clin. Invest. 116, 3171–3182 (2006).
Nieborowska-Skorska, M. Et al. Role of signal transducer and activator of transcription 5 in nucleophosmin/anaplastic lymphoma kinase-mediated malignant transformation of lymphoid cells. Cancer Res. 61, 6517–6523 (2001).
Ruchatz, H., Coluccia, A. M., Stano, P., Marchesi, E. & Gambacorti-Passerini, C. Constitutive activation of Jak2 contributes to proliferation and resistance to apoptosis in NPM/ALK-transformed cells. Exp. Hematol. 31, 309–315 (2003).
Zhang, Q., Wang, H. Y., Liu, X. & Wasik, M. A. STAT5A is epigenetically silenced by the tyrosine kinase NPM1–ALK and acts as a tumor suppressor by reciprocally inhibiting NPM1–ALK expression. Nature Med. 13, 1341–1348 (2007).
Slupianek, A. Et al. Role of phosphatidylinositol 3-kinase-Akt pathway in nucleophosmin/anaplastic lymphoma kinase-mediated lymphomagenesis. Cancer Res. 61, 2194–2199 (2001).
Rassidakis, G. Z. Et al. Inhibition of Akt increases p27Kip1 levels and induces cell cycle arrest in anaplastic large cell lymphoma. Blood 105, 827–829 (2005).
Slupianek, A. & Skorski, T. NPM/ALK downregulates p27Kip1 in a PI-3K-dependent manner. Exp. Hematol. 32, 1265–1271 (2004).
Marzec, M. Et al. Oncogenic tyrosine kinase NPM/ALK induces activation of the rapamycin-sensitive mTOR signaling pathway. Oncogene 26, 5606–5614 (2007).
Vega, F. Et al. Activation of mammalian target of rapamycin signaling pathway contributes to tumor cell survival in anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Cancer Res. 66, 6589–6597 (2006).
Armstrong, F. Et al. Differential effects of X–ALK fusion proteins on proliferation, transformation, and invasion properties of NIH3T3 cells. Oncogene 23, 6071–6082 (2004).
Motegi, A., Fujimoto, J., Kotani, M., Sakuraba, H. & Yamamoto, T. ALK receptor tyrosine kinase promotes cell growth and neurite outgrowth. J. Cell Sci. 117, 3319–3329 (2004).
Horie, R. Et al. The NPM–ALK oncoprotein abrogates CD30 signaling and constitutive NF-κB activation in anaplastic large cell lymphoma. Cancer Cell 5, 353–364 (2004).
Colomba, A. Et al. Activation of Rac1 and the exchange factor Vav3 are involved in NPM–ALK signaling in anaplastic large cell lymphomas. Oncogene (2007).
Bonzheim, I. Et al. Anaplastic large cell lymphomas lack the expression of T-cell receptor molecules or molecules of proximal T-cell receptor signaling. Blood 104, 3358–3360 (2004).
Kasprzycka, M., Marzec, M., Liu, X., Zhang, Q. & Wasik, M. A. Nucleophosmin/anaplastic lymphoma kinase (NPM/ALK) oncoprotein induces the T regulatory cell phenotype by activating STAT3. Proc. Natl Acad. Sci. USA 103, 9964–9969 (2006).
Roncador, G. Et al. FOXP3, a selective marker for a subset of adult T-cell leukaemia/lymphoma. Leukemia 19, 2247–2253 (2005).
Hsu, F. Y., Johnston, P. B., Burke, K. A. & Zhao, Y. The expression of CD30 in anaplastic large cell lymphoma is regulated by nucleophosmin-anaplastic lymphoma kinase-mediated JunB level in a cell type-specific manner. Cancer Res. 66, 9002–9008 (2006). This paper demonstrates for the first time the functional relationship between NPM–ALK and CD30, identifying JUNB as the mediator of NPM–ALK-dependent CD30 transcriptional regulation.
Watanabe, M. Et al. JunB induced by constitutive CD30-extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase signaling activates the CD30 promoter in anaplastic large cell lymphoma and Reed–Sternberg cells of Hodgkin lymphoma. Cancer Res. 65, 7628–7634 (2005).
Horie, R. Et al. Ligand-independent signaling by overexpressed CD30 drives NF-κB activation in Hodgkin–Reed–Sternberg cells. Oncogene 21, 2493–2503 (2002).
Wright, C. W., Rumble, J. M. & Duckett, C. S. CD30 activates both the canonical and alternative NF-κB pathways in anaplastic large cell lymphoma cells. J. Biol. Chem. 282, 10252–10262 (2007).
Ohno, H., Nishikori, M., Maesako, Y. & Haga, H. Reappraisal of BCL3 as a molecular marker of anaplastic large cell lymphoma. Int. J. Hematol. 82, 397–405 (2005).
Thompson, M. A. Et al. Differential gene expression in anaplastic lymphoma kinase-positive and anaplastic lymphoma kinase-negative anaplastic large cell lymphomas. Hum. Pathol. 36, 494–504 (2005).
Trempat, P. Et al. Gene expression profiling in anaplastic large cell lymphoma and Hodgkin's disease. Leuk. Lymphoma 45, 2001–2006 (2004).
Lamant, L. Et al. Gene-expression profiling of systemic anaplastic large-cell lymphoma reveals differences based on ALK status and two distinct morphologic ALK+ subtypes. Blood 109, 2156–2164 (2007).
Piccaluga, P. P. Et al. Gene expression analysis of peripheral T cell lymphoma, unspecified, reveals distinct profiles and new potential therapeutic targets. J. Clin. Invest. 117, 823–834 (2007).
Rush, J. Et al. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nature Biotechnol. 23, 94–101 (2005).
Elenitoba-Johnson, K. S. Et al. Proteomic identification of oncogenic chromosomal translocation partners encoding chimeric anaplastic lymphoma kinase fusion proteins. Proc. Natl Acad. Sci. USA 103, 7402–7407 (2006).
Crockett, D. K., Lin, Z., Elenitoba-Johnson, K. S. & Lim, M. S. Identification of NPM–ALK interacting proteins by tandem mass spectrometry. Oncogene 23, 2617–2629 (2004).
Lim, M. S. & Elenitoba-Johnson, K. S. Mass spectrometry-based proteomic studies of human anaplastic large cell lymphoma. Mol. Cell Proteomics 5, 1787–1798 (2006).
Fanale, M. A. & Younes, A. Monoclonal antibodies in the treatment of non-Hodgkin's lymphoma. Drugs 67, 333–350 (2007).
Li, R. & Morris, S. W. Development of anaplastic lymphoma kinase (ALK) small-molecule inhibitors for cancer therapy. Med. Res. Rev. 10 August 2007 (doi: 10.1002/med.20109).
Galkin, A. V. Et al. Identification of NVP-TAE684, a potent, selective, and efficacious inhibitor of NPM–ALK. Proc. Natl Acad. Sci. USA 104, 270–275 (2007).
Wan, W. Et al. Anaplastic lymphoma kinase activity is essential for the proliferation and survival of anaplastic large-cell lymphoma cells. Blood 107, 1617–1623 (2006).
Zou, H. Y. Et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 67, 4408–4417 (2007). This paper, and the preceding two, shows the development of the first small molecules with selective ALK inhibitory activity, which result in G1 cell-cycle arrest and inactivation of ERK1 and ERK2, STAT3 and Akt signaling pathways. These results suggest a therapeutic application for ALK-positive ALCL and possibly other solid and haematological tumours in which ALK activation is implicated in their pathogenesis.
Piva, R. Et al. Ablation of oncogenic ALK is a viable therapeutic approach for anaplastic large-cell lymphomas. Blood 107, 689–697 (2006). Proof of principle that ALK is a viable target for therapeutic intervention and that its inactivation might be a pivotal approach for the treatment of ALK lymphomas.
Ait-Tahar, K. Et al. B and CTL responses to the ALK protein in patients with ALK-positive ALCL. Int. J. Cancer 118, 688–695 (2006).
Passoni, L. Et al. In vivo T-cell immune response against anaplastic lymphoma kinase in patients with anaplastic large cell lymphomas. Haematologica 91, 48–55 (2006).
Pulford, K. Et al. Immune response to the ALK oncogenic tyrosine kinase in patients with anaplastic large-cell lymphoma. Blood 96, 1605–1607 (2000).
Lollini, P. L., Cavallo, F., Nanni, P. & Forni, G. Vaccines for tumour prevention. Nature Rev. Cancer 6, 204–216 (2006).
Kim, D. H. & Rossi, J. J. Strategies for silencing human disease using RNA interference. Nature Rev. Genet. 8, 173–184 (2007).
Englund, C. Et al. Jeb signals through the Alk receptor tyrosine kinase to drive visceral muscle fusion. Nature 425, 512–516 (2003).
Lee, H. H., Norris, A., Weiss, J. B. & Frasch, M. Jelly belly protein activates the receptor tyrosine kinase Alk to specify visceral muscle pioneers. Nature 425, 507–512 (2003). This paper and the preceding one are the first description of a physiological role of an ALK homologue in Drosophila melanogaster.
Bazigou, E. Et al. Anterograde Jelly belly and Alk receptor tyrosine kinase signaling mediates retinal axon targeting in Drosophila. Cell 128, 961–975 (2007).
Liao, E. H., Hung., W., Abrams, B. & Zhen, M. An SCF-like ubiquitin ligase complex that controls presynaptic differentiation. Nature 430, 345–350 (2004).
Vernersson, E. Et al. Characterization of the expression of the ALK receptor tyrosine kinase in mice. Gene Expr. Patterns 6, 448–461 (2006).
Morris, S. W. Et al. ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin's lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK). Oncogene 14, 2175–2188 (1997).
Iwahara, T. Et al. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene 14, 439–449 (1997).
Souttou, B., Carvalho, N. B., Raulais, D. & Vigny, M. Activation of anaplastic lymphoma kinase receptor tyrosine kinase induces neuronal differentiation through the mitogen-activated protein kinase pathway. J. Biol. Chem. 276, 9526–9531 (2001).
Piccinini, G. Et al. A ligand-inducible epidermal growth factor receptor/anaplastic lymphoma kinase chimera promotes mitogenesis and transforming properties in 3T3 cells. J. Biol. Chem. 277, 22231–22239 (2002).
Bilsland, J. G. Et al. Behavioral and neurochemical alterations in mice deficient in anaplastic lymphoma kinase suggest therapeutic potential for psychiatric indications. Neuropsychopharmacology 9 May 2007 (doi: 10.1038/sj.Npp.1301446).